Behavioral and genetic dissection of a mouse model for advanced sleep phase syndrome
- PMID: 21203370
- PMCID: PMC3001793
- DOI: 10.1093/sleep/34.1.39
Behavioral and genetic dissection of a mouse model for advanced sleep phase syndrome
Abstract
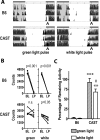
Study objective: The adaptive value of the endogenous circadian clock arises from its ability to synchronize (i.e., entrain) to external light-dark (LD) cycles at an appropriate phase. Studies have suggested that advanced circadian phase alignment might result from shortening of the period length of the clock. Here we explore mechanisms that contribute to an early activity phase in CAST/EiJ (CAST) mice.
Methods: We investigated circadian rhythms of wheel-running activity in C57BL/6J (B6), CAST and 2 strains of B6.CAST congenic mice, which carry CAST segments introgressed in a B6 genome.
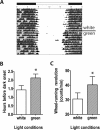
Results: When entrained, all CAST mice initiate daily activity several hours earlier than normal mice. This difference could not be explained by alterations in the endogenous period, as activity onset did not correlate with period length. However, the photic phase-shifting responses in these mice were phase-lagged by 3 hours relative to their activity. Attenuated light masking responses were also found in CAST mice, which allow for activity normally inhibited by light. A previously identified quantitative trait locus (QTL), Era1, which contributes to the early activity trait, was confirmed and refined here using two B6.CAST congenic strains. Surprisingly, these B6.CAST mice exhibited longer rather than shorter endogenous periods, further demonstrating that the advanced phase in these mice is not due to alterations in period.
Conclusions: CAST mice have an advanced activity phase similar to human advanced sleep phase syndrome. This advanced phase is not due to its shorter period length or smaller light-induced phase shifts, but appears to be related to both light masking and altered coupling of the circadian pacemaker with various outputs. Lastly, a QTL influencing this trait was confirmed and narrowed using congenic mice as a first step toward gene identification.
Keywords: Era1; Phase angle; mouse genetics; phase response curve; quantitative trait loci.
Figures





References
-
- Ando K, Kripke DF, Ancoli-Israel S. Delayed and advanced sleep phase symptoms. Isr J Psychiatry Relat Sci. 2002;39:11–8. - PubMed
-
- Baker SK, Zee PC. Circadian disorders of the sleep-wake cycle. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: WB Saunders; 2000. pp. 606–14.
-
- Zisapel N. Circadian rhythm sleep disorders: pathophysiology and potential approaches to management. CNS Drugs. 2001;15:311–28. - PubMed
-
- Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int. 2003;20:741–74. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

