Molecular basis of differential B-pentamer stability of Shiga toxins 1 and 2
- PMID: 21203383
- PMCID: PMC3010993
- DOI: 10.1371/journal.pone.0015153
Molecular basis of differential B-pentamer stability of Shiga toxins 1 and 2
Abstract
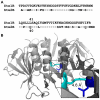
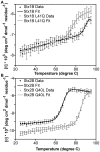
Escherichia coli strain O157:H7 is a major cause of food poisoning that can result in severe diarrhea and, in some cases, renal failure. The pathogenesis of E. coli O157:H7 is in large part due to the production of Shiga toxin (Stx), an AB(5) toxin that consists of a ribosomal RNA-cleaving A-subunit surrounded by a pentamer of receptor-binding B subunits. There are two major isoforms, Stx1 and Stx2, which differ dramatically in potency despite having 57% sequence identity. Animal studies and epidemiological studies show Stx2 is associated with more severe disease. Although the molecular basis of this difference is unknown, data suggest it is associated with the B-subunit. Mass spectrometry studies have suggested differential B-pentamer stability between Stx1 and Stx2. We have examined the relative stability of the B-pentamers in solution. Analytical ultracentrifugation using purified B-subunits demonstrates that Stx2B, the more deadly isoform, shows decreased pentamer stability compared to Stx1B (EC(50) = 2.3 µM vs. EC(50) = 0.043 µM for Stx1B). X-ray crystal structures of Stx1B and Stx2B identified a glutamine in Stx2 (versus leucine in Stx1) within the otherwise strongly hydrophobic interface between B-subunits. Interchanging these residues switches the stability phenotype of the B-pentamers of Stx1 and Stx2, as demonstrated by analytical ultracentrifugation and circular dichroism. These studies demonstrate a profound difference in stability of the B-pentamers in Stx1 and Stx2, illustrate the mechanistic basis for this differential stability, and provide novel reagents to test the basis for differential pathogenicity of these toxins.
Conflict of interest statement
Figures




References
-
- Centers for Disease Control and Prevention. Disease listing, enterohemorrhagic E. coli. 2005.
-
- Mead PS, Griffin PM. Escherichia coli O157:H7. Lancet. 1998;352(9135):1207–1212. - PubMed
-
- Boyce TG, Swerdlow DL, Griffin PM. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333(6):364–368. - PubMed
-
- Siegler RL. The hemolytic uremic syndrome. Pediatr Clin North Am. 1995;42(6):1505–1529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

