RAF kinase activity regulates neuroepithelial cell proliferation and neuronal progenitor cell differentiation during early inner ear development
- PMID: 21203386
- PMCID: PMC3010996
- DOI: 10.1371/journal.pone.0014435
RAF kinase activity regulates neuroepithelial cell proliferation and neuronal progenitor cell differentiation during early inner ear development
Abstract
Background: Early inner ear development requires the strict regulation of cell proliferation, survival, migration and differentiation, coordinated by the concerted action of extrinsic and intrinsic factors. Deregulation of these processes is associated with embryonic malformations and deafness. We have shown that insulin-like growth factor I (IGF-I) plays a key role in embryonic and postnatal otic development by triggering the activation of intracellular lipid and protein kinases. RAF kinases are serine/threonine kinases that regulate the highly conserved RAS-RAF-MEK-ERK signaling cascade involved in transducing the signals from extracellular growth factors to the nucleus. However, the regulation of RAF kinase activity by growth factors during development is complex and still not fully understood.
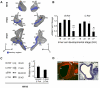
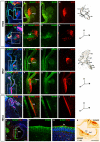
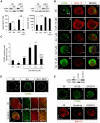
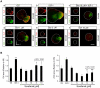
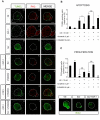
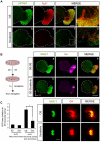
Methodology/principal findings: By using a combination of qRT-PCR, Western blotting, immunohistochemistry and in situ hybridization, we show that C-RAF and B-RAF are expressed during the early development of the chicken inner ear in specific spatiotemporal patterns. Moreover, later in development B-RAF expression is associated to hair cells in the sensory patches. Experiments in ex vivo cultures of otic vesicle explants demonstrate that the influence of IGF-I on proliferation but not survival depends on RAF kinase activating the MEK-ERK phosphorylation cascade. With the specific RAF inhibitor Sorafenib, we show that blocking RAF activity in organotypic cultures increases apoptosis and diminishes the rate of cell proliferation in the otic epithelia, as well as severely impairing neurogenesis of the acoustic-vestibular ganglion (AVG) and neuron maturation.
Conclusions/significance: We conclude that RAF kinase activity is essential to establish the balance between cell proliferation and death in neuroepithelial otic precursors, and for otic neuron differentiation and axonal growth at the AVG.
Conflict of interest statement
Figures

References
-
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. - PubMed
-
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. - PubMed
-
- León Y, Sánchez-Galiano S, Gorospe I. Programmed cell death in the development of the vertebrate inner ear. Apoptosis. 2004;9:255–264. - PubMed
-
- Sanchez-Calderon H, Milo M, Leon Y, Varela-Nieto I. A network of growth and transcription factors controls neuronal differentation and survival in the developing ear. Int J Dev Biol. 2007;51:557–570. - PubMed
-
- Davies D. Temporal and spatial regulation of alpha6 integrin expression during the development of the cochlear-vestibular ganglion. J Comp Neurol. 2007;502:673–682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

