In vivo imaging of HIF-active tumors by an oxygen-dependent degradation protein probe with an interchangeable labeling system
- PMID: 21203417
- PMCID: PMC3009742
- DOI: 10.1371/journal.pone.0015736
In vivo imaging of HIF-active tumors by an oxygen-dependent degradation protein probe with an interchangeable labeling system
Abstract
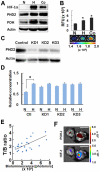
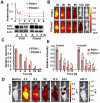
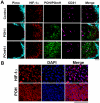
Hypoxia-inducible factor (HIF) functions as a master transcriptional regulator for adaptation to hypoxia by inducing adaptive changes in gene expression for regulation of proliferation, angiogenesis, apoptosis and energy metabolism. Cancers with high expression of the alpha subunit of HIF (HIFα) are often malignant and treatment-resistant. Therefore, the development of a molecular probe that can detect HIF activity has great potential value for monitoring tumor hypoxia. HIF prolyl hydroxylases (HPHDs) act as oxygen sensors that regulate the fate of HIFα protein through its oxygen-dependent degradation (ODD) domain. We constructed a recombinant protein PTD-ODD-HaloTag (POH) that is under the same ODD regulation as HIFα and contains protein transduction domain (PTD) and an interchangeable labeling system. Administration of near-infrared fluorescently labeled POH (POH-N) to mouse models of cancers allowed successful monitoring of HIF-active regions. Immunohistochemical analysis for intratumoral localization of POH probe revealed its specificity to HIF-active cells. Furthermore, lack of the PTD domain or a point mutation in the ODD domain abrogated the specificity of POH-N to HIF-active cells. Overall results indicate that POH is a practical probe specific to HIF-active cell in cancers and suggest its large potential for imaging and targeting of HIF-related diseases.
Conflict of interest statement
Figures



References
-
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. - PubMed
-
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. - PubMed
-
- Semenza GL. Hypoxia-inducible factor 1 and cancer pathogenesis. IUBMB Life. 2008;60:591–597. - PubMed
-
- Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115–128. - PubMed
-
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

