Self-mating in the definitive host potentiates clonal outbreaks of the apicomplexan parasites Sarcocystis neurona and Toxoplasma gondii
- PMID: 21203443
- PMCID: PMC3009688
- DOI: 10.1371/journal.pgen.1001261
Self-mating in the definitive host potentiates clonal outbreaks of the apicomplexan parasites Sarcocystis neurona and Toxoplasma gondii
Abstract
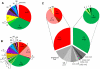
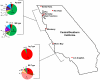
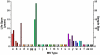
Tissue-encysting coccidia, including Toxoplasma gondii and Sarcocystis neurona, are heterogamous parasites with sexual and asexual life stages in definitive and intermediate hosts, respectively. During its sexual life stage, T. gondii reproduces either by genetic out-crossing or via clonal amplification of a single strain through self-mating. Out-crossing has been experimentally verified as a potent mechanism capable of producing offspring possessing a range of adaptive and virulence potentials. In contrast, selfing and other life history traits, such as asexual expansion of tissue-cysts by oral transmission among intermediate hosts, have been proposed to explain the genetic basis for the clonal population structure of T. gondii. In this study, we investigated the contributing roles self-mating and sexual recombination play in nature to maintain clonal population structures and produce or expand parasite clones capable of causing disease epidemics for two tissue encysting parasites. We applied high-resolution genotyping against strains isolated from a T. gondii waterborne outbreak that caused symptomatic disease in 155 immune-competent people in Brazil and a S. neurona outbreak that resulted in a mass mortality event in Southern sea otters. In both cases, a single, genetically distinct clone was found infecting outbreak-exposed individuals. Furthermore, the T. gondii outbreak clone was one of several apparently recombinant progeny recovered from the local environment. Since oocysts or sporocysts were the infectious form implicated in each outbreak, the expansion of the epidemic clone can be explained by self-mating. The results also show that out-crossing preceded selfing to produce the virulent T. gondii clone. For the tissue encysting coccidia, self-mating exists as a key adaptation potentiating the epidemic expansion and transmission of newly emerged parasite clones that can profoundly shape parasite population genetic structures or cause devastating disease outbreaks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Li W, Raoult D, Fournier PE. Bacterial strain typing in the genomic era. FEMS Microbiol Rev. 2009;33:892–916. - PubMed
-
- Feil EJ, Spratt BG. Recombination and the population structures of bacterial pathogens. Annu Rev Microbiol. 2001;55:561–590. - PubMed
-
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. - PubMed
-
- Powers C, DeFilippis V, Malouli D, Fruh K. Cytomegalovirus immune evasion. Curr Top Microbiol Immunol. 2008;325:333–359. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

