Detection and functional characterization of a 215 amino acid N-terminal extension in the Xanthomonas type III effector XopD
- PMID: 21203472
- PMCID: PMC3008746
- DOI: 10.1371/journal.pone.0015773
Detection and functional characterization of a 215 amino acid N-terminal extension in the Xanthomonas type III effector XopD
Retraction in
-
Retraction: Detection and Functional Characterization of a 215 Amino Acid N-Terminal Extension in the Xanthomonas Type III Effector XopD.PLoS One. 2018 Jan 2;13(1):e0190773. doi: 10.1371/journal.pone.0190773. eCollection 2018. PLoS One. 2018. PMID: 29293678 Free PMC article. No abstract available.
Abstract
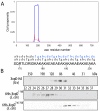
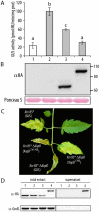
During evolution, pathogens have developed a variety of strategies to suppress plant-triggered immunity and promote successful infection. In Gram-negative phytopathogenic bacteria, the so-called type III protein secretion system works as a molecular syringe to inject type III effectors (T3Es) into plant cells. The XopD T3E from the strain 85-10 of Xanthomonas campestris pathovar vesicatoria (Xcv) delays the onset of symptom development and alters basal defence responses to promote pathogen growth in infected tomato leaves. XopD was previously described as a modular protein that contains (i) an N-terminal DNA-binding domain (DBD), (ii) two tandemly repeated EAR (ERF-associated amphiphillic repression) motifs involved in transcriptional repression, and (iii) a C-terminal cysteine protease domain, involved in release of SUMO (small ubiquitin-like modifier) from SUMO-modified proteins. Here, we show that the XopD protein that is produced and secreted by Xcv presents an additional N-terminal extension of 215 amino acids. Closer analysis of this newly identified N-terminal domain shows a low complexity region rich in lysine, alanine and glutamic acid residues (KAE-rich) with high propensity to form coiled-coil structures that confers to XopD the ability to form dimers when expressed in E. coli. The full length XopD protein identified in this study (XopD(1-760)) displays stronger repression of the XopD plant target promoter PR1, as compared to the XopD version annotated in the public databases (XopD(216-760)). Furthermore, the N-terminal extension of XopD, which is absent in XopD(216-760), is essential for XopD type III-dependent secretion and, therefore, for complementation of an Xcv mutant strain deleted from XopD in its ability to delay symptom development in tomato susceptible cultivars. The identification of the complete sequence of XopD opens new perspectives for future studies on the XopD protein and its virulence-associated functions in planta.
Conflict of interest statement
Figures




References
-
- Zipfel C. Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol. 2008;20:10–16. - PubMed
-
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. - PubMed
-
- Loake G, Grant M. Salicylic acid in plant defence: the players and protagonists. Curr Opin Plant Biol. 2007;10:466–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

