Placebos without deception: a randomized controlled trial in irritable bowel syndrome
- PMID: 21203519
- PMCID: PMC3008733
- DOI: 10.1371/journal.pone.0015591
Placebos without deception: a randomized controlled trial in irritable bowel syndrome
Abstract
Background: Placebo treatment can significantly influence subjective symptoms. However, it is widely believed that response to placebo requires concealment or deception. We tested whether open-label placebo (non-deceptive and non-concealed administration) is superior to a no-treatment control with matched patient-provider interactions in the treatment of irritable bowel syndrome (IBS).
Methods: Two-group, randomized, controlled three week trial (August 2009-April 2010) conducted at a single academic center, involving 80 primarily female (70%) patients, mean age 47 ± 18 with IBS diagnosed by Rome III criteria and with a score ≥ 150 on the IBS Symptom Severity Scale (IBS-SSS). Patients were randomized to either open-label placebo pills presented as "placebo pills made of an inert substance, like sugar pills, that have been shown in clinical studies to produce significant improvement in IBS symptoms through mind-body self-healing processes" or no-treatment controls with the same quality of interaction with providers. The primary outcome was IBS Global Improvement Scale (IBS-GIS). Secondary measures were IBS Symptom Severity Scale (IBS-SSS), IBS Adequate Relief (IBS-AR) and IBS Quality of Life (IBS-QoL).
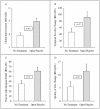
Findings: Open-label placebo produced significantly higher mean (±SD) global improvement scores (IBS-GIS) at both 11-day midpoint (5.2 ± 1.0 vs. 4.0 ± 1.1, p<.001) and at 21-day endpoint (5.0 ± 1.5 vs. 3.9 ± 1.3, p = .002). Significant results were also observed at both time points for reduced symptom severity (IBS-SSS, p = .008 and p = .03) and adequate relief (IBS-AR, p = .02 and p = .03); and a trend favoring open-label placebo was observed for quality of life (IBS-QoL) at the 21-day endpoint (p = .08).
Conclusion: Placebos administered without deception may be an effective treatment for IBS. Further research is warranted in IBS, and perhaps other conditions, to elucidate whether physicians can benefit patients using placebos consistent with informed consent.
Trial registration: ClinicalTrials.gov NCT01010191.
Conflict of interest statement
Figures
References
-
- Miller FG, Colloca L. The legitimacy of placebo treatments in clinical practice: evidence and ethics. American Journal of Bioethics. 2009;9:39–47. - PubMed
-
- American Medical Association Placebo Use in Clinical Practice. 2006. CEJA Report 2-I-2006. Accessed online 05/16/07 at http://www.ama assn.org/ama1/pub/upload/mm/369/ceja_recs_2i06.pdf. Assessed 2010 January 5.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous