Biology and function of neuroimmune semaphorins 4A and 4D
- PMID: 21203905
- PMCID: PMC3366695
- DOI: 10.1007/s12026-010-8201-y
Biology and function of neuroimmune semaphorins 4A and 4D
Abstract
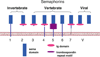
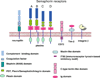
Semaphorins belong to a family of membrane-bound and secreted molecules that regulate the functional activity of axons in the nervous system. Sema4A and Sema4D were the first semaphorins also found to be expressed in immune cells and were, therefore, termed "immune semaphorins". It is known that Sema4A has three functional receptors, namely Plexin D1, Plexin B1, and Tim-2, whereas Sema4D binds to Plexin B1 and CD72. Recent studies suggest that immune semaphorins play critical roles in many physiological and pathological processes and such. In this review, we summarize the current knowledge on the biology of neuroimmune semaphorins and their corresponding receptors, their distribution in organs and tissues, function in the immune response, and critical regulatory roles in various diseases.
Figures


References
-
- Kumanogoh A, Kikutani H. Immune semaphorins: a new area of semaphorin research. J Cell Sci. 2003;116:3463–3470. - PubMed
-
- Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. - PubMed
-
- Koppel AM, Feiner L, Kobayashi H, Raper JA. A 70 amino acid region within the semaphorin domain activates specific cellular response of semaphorin family members. Neuron. 1997;19:531–537. - PubMed
-
- Gherardi E, Love CA, Esnouf RM, Jones EY. The sema domain. Curr Opin Struct Biol. 2004;14:669–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

