HER2: biology, detection, and clinical implications
- PMID: 21204711
- PMCID: PMC3242418
- DOI: 10.5858/2010-0454-RAR.1
HER2: biology, detection, and clinical implications
Abstract
Context: HER2 is a membrane tyrosine kinase and oncogene that is overexpressed and gene amplified in about 20% of breast cancers. When activated it provides the cell with potent proliferative and antiapoptosis signals and it is the major driver of tumor development and progression for this subset of breast cancer. When shown to be overexpressed or amplified by appropriate methods, HER2 is a valuable treatment target.
Objectives: To review the basic biology of the HER2 signaling network, to discuss various approved methods for its detection in clinical specimens, and to describe the impressive results of therapies targeting HER2.
Data sources: Selected literature searchable on PubMed as well as older studies revealed by the literature review were reviewed.
Conclusion: HER2 is an important member of a complex signaling network and when gene amplified, it results in an aggressive subtype of breast cancer. Patients with tumors found to overexpress HER2 protein or to be amplified for the gene are candidates for therapy that significantly reduces mortality.
Conflict of interest statement
The authors have no relevant financial interest in the products or companies described in this article.
Figures

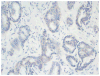


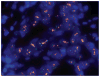



References
-
- King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985 Sep 6;229(4717):974–976. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. - PubMed
-
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998 May 16;351(9114):1451–1467. - PubMed
-
- Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009 Dec 1;27(34):5685–5692. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005 Oct 20;353(16):1659–1672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
