In vitro and in vivo evaluation of ⁶⁴Cu-radiolabeled KCCYSL peptides for targeting epidermal growth factor receptor-2 in breast carcinomas
- PMID: 21204764
- PMCID: PMC3026654
- DOI: 10.1089/cbr.2010.0820
In vitro and in vivo evaluation of ⁶⁴Cu-radiolabeled KCCYSL peptides for targeting epidermal growth factor receptor-2 in breast carcinomas
Abstract
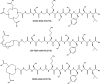
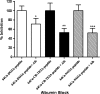
Epidermal growth factor receptor-2 (EGFR-2) has been implicated in the pathogenesis of breast and other carcinomas. In this report, we tested the ability of a bacteriophage selected peptide KCCYSL, radiolabeled with ⁶⁴Cu, to image EGFR-2 expressing breast tumors in vivo by positron emission tomography (PET). We evaluated and compared the in vivo tissue distribution and imaging properties of ⁶⁴Cu-X-(Gly-Ser-Gly)-KCCYSL peptide (X = 1,4,7,10, tetraazacyclododecane-N,N',N'',N'''-tetracetic acid, [DOTA] 1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4,11-diacetic acid [CB-TE2A], and 1,4,7-triazacyclononane-1,4,7-triacetic acid [NOTA] chelators) in a human breast cancer xenograft mouse model using dual modality PET and computed tomography (CT). The synthesized peptides DO3A-GSG-KCCYSL, CB-TE2A-GSG-KCCYSL, and NO2A-GSG-KCCYSL were purified, radiolabeled with ⁶⁴Cu, and evaluated for human breast cancer cell (MDA-MB-435) binding, 50% inhibitory concentration, and serum stability. In vivo pharmacokinetic and small animal PET/CT imaging studies were performed using SCID mice bearing MDA-MB-435 xenografts. The radiolabeled peptides bound with an 50% inhibitory concentration of 42.0 ± 10.2 nM (DO3A), 31 ± 7.9 nM (CB-TE2A), and 44.2 ± 6.6 nM (NO2A) to cultured MDA-MB-435 cells. All of the radiolabeled peptides were stable in vitro. The tumor uptake of DO3A, CB-TE2A, and NO2A peptides were 0.73 ± 0.15 percent injected dose per gram (%ID/g), 0.64 ± 0.08%ID/g, and 0.52 ± 0.04%ID/g, respectively at 1 hour. Liver uptake for the ⁶⁴Cu-DO3A-peptide (1.68 ± 0.42%ID/g) was more than that of the ⁶⁴Cu-CB-TE2A-peptide (0.52 ± 0.02% ID/g) and ⁶⁴Cu-NO2A-peptide (0.48 ± 0.05%ID/g) at 2 hours. PET/CT studies revealed successful tumor uptake of ⁶⁴Cu-peptides at 2 hours postinjection. In vivo kidney retention was observed with all of the radiolabeled peptides. The optimization of bifunctional chelators improves the pharmacokinetic properties of the ⁶⁴Cu-labeled GSG-KCCYSL peptide, which enables the selection of a suitable peptide homolog as a PET imaging agent for EGFR-2 expressing breast carcinomas.
Figures

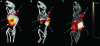
Similar articles
-
(64)Cu-labeled peptide for PET of breast carcinomas expressing the Thomsen-Friedenreich carbohydrate antigen.J Nucl Med. 2011 Nov;52(11):1819-26. doi: 10.2967/jnumed.111.093716. Epub 2011 Oct 7. J Nucl Med. 2011. PMID: 21984800
-
In vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using 64Cu bombesin analogs: side-by-side comparison of the CB-TE2A and DOTA chelation systems.J Nucl Med. 2007 Aug;48(8):1327-37. doi: 10.2967/jnumed.107.039487. Epub 2007 Jul 13. J Nucl Med. 2007. PMID: 17631556
-
Evaluation of an 111In-radiolabeled peptide as a targeting and imaging agent for ErbB-2 receptor expressing breast carcinomas.Clin Cancer Res. 2007 Oct 15;13(20):6070-9. doi: 10.1158/1078-0432.CCR-07-0160. Clin Cancer Res. 2007. PMID: 17947470
-
64Cu-1,4,7-Triazacyclononane-1,4-diacetic acid-9-aminonanoic acid-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2.2011 Jan 2 [updated 2011 Jan 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Jan 2 [updated 2011 Jan 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21290625 Free Books & Documents. Review.
-
64Cu-1,4,7-Triazacyclononane-1,4-diacetic acid-6-aminohexanoic acid-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2.2011 Jan 2 [updated 2011 Jan 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Jan 2 [updated 2011 Jan 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21290624 Free Books & Documents. Review.
Cited by
-
Cyclotron Production of High-Specific Activity 55Co and In Vivo Evaluation of the Stability of 55Co Metal-Chelate-Peptide Complexes.Mol Imaging. 2015;14(10):526-33. doi: 10.2310/7290.2015.00025. Mol Imaging. 2015. PMID: 26505224 Free PMC article.
-
Peptides Targeting HER2-Positive Breast Cancer Cells and Applications in Tumor Imaging and Delivery of Chemotherapeutics.Nanomaterials (Basel). 2023 Sep 1;13(17):2476. doi: 10.3390/nano13172476. Nanomaterials (Basel). 2023. PMID: 37686984 Free PMC article. Review.
-
Preclinical research progress in HER2-targeted small-molecule probes for breast cancer.Radiologie (Heidelb). 2024 Nov;64(Suppl 1):47-53. doi: 10.1007/s00117-024-01338-5. Epub 2024 Jul 22. Radiologie (Heidelb). 2024. PMID: 39039211 Free PMC article. Review.
-
Development of the Tumor-Specific Antigen-Derived Synthetic Peptides as Potential Candidates for Targeting Breast and Other Possible Human Carcinomas.Molecules. 2019 Aug 29;24(17):3142. doi: 10.3390/molecules24173142. Molecules. 2019. PMID: 31470531 Free PMC article.
-
Selection of Cancer Stem Cell-Targeting Agents Using Bacteriophage Display.Methods Mol Biol. 2022;2394:787-810. doi: 10.1007/978-1-0716-1811-0_41. Methods Mol Biol. 2022. PMID: 35094358
References
-
- Ullrich A. Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203. - PubMed
-
- Hung MC. Lau YK. Basic science of HER2/neu: A review. Semin Oncol. 1999;26(Suppl):51. - PubMed
-
- Press MF. Cordon-Cardo C. Slamon DJ. Expression of the Her2/neuproto-oncogene in normal human and adult and fetal tissues. Oncogene. 1990;5:53. - PubMed
-
- Ross J. Fletcher JA. The Her2/neu oncogene in breast cancer: Prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413. - PubMed
-
- Hynes NE. Stern DF. The biology of ErbB-2/neu/Her-2 and its role in cancer. Biochimica et Biophysica Acta. 1994;1198:165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

