Male infertility-linked point mutation disrupts the Ca2+ oscillation-inducing and PIP(2) hydrolysis activity of sperm PLCζ
- PMID: 21204786
- PMCID: PMC3195387
- DOI: 10.1042/BJ20101772
Male infertility-linked point mutation disrupts the Ca2+ oscillation-inducing and PIP(2) hydrolysis activity of sperm PLCζ
Erratum in
- Biochem J. 2011 May 1;435(3):791. Nounesis, Georg [corrected to Nounesis, George]
Abstract
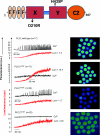
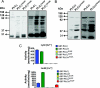
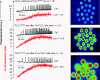
A male infertility-linked human PLCζ (phospholipase Cζ) mutation introduced into mouse PLCζ completely abolishes both in vitro PIP(2) (phosphatidylinositol 4,5-bisphosphate) hydrolysis activity and the ability to trigger in vivo Ca2+ oscillations in mouse eggs. Wild-type PLCζ initiated a normal pattern of Ca2+ oscillations in eggs in the presence of 10-fold higher mutant PLCζ, suggesting that infertility is not mediated by a dominant-negative mechanism.
© The Authors Journal compilation © 2011 Biochemical Society
Figures
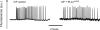
References
-
- Stricker S. A. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 1999;211:157–176. - PubMed
-
- Runft L. L., Jaffe L. A., Mehlmann L. M. Egg activation at fertilization: where it all begins. Dev. Biol. 2002;245:237–254. - PubMed
-
- Swann K. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development. 1990;110:1295–1302. - PubMed
-
- Schultz R. M., Kopf G. S. Molecular basis of mammalian egg activation. Curr. Top. Dev. Biol. 1995;30:21–62. - PubMed
-
- Jones K. T. Ca2+ oscillations in the activation of the egg and development of the embryo in mammals. Int. J. Dev. Biol. 1998;42:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

