Comparative pharmacodynamic and pharmacokinetic characteristics of subcutaneous insulin glulisine and insulin aspart prior to a standard meal in obese subjects with type 2 diabetes
- PMID: 21205115
- PMCID: PMC3132447
- DOI: 10.1111/j.1463-1326.2010.01343.x
Comparative pharmacodynamic and pharmacokinetic characteristics of subcutaneous insulin glulisine and insulin aspart prior to a standard meal in obese subjects with type 2 diabetes
Abstract
Aims: A multinational, randomized, double-blind, two-way crossover trial to compare the pharmacokinetic and pharmacodynamic properties of bolus, subcutaneously administered insulin glulisine (glulisine) and insulin aspart (aspart) in insulin-naÏve, obese subjects with type 2 diabetes.
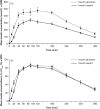
Methods: Thirty subjects [9/21 females/males; mean ± SD age: 60.7 ± 7.7 years; body mass index (BMI): 33.5 ± 3.3 kg/m(2) ; duration of diabetes: 6.8 ± 4.6 years; HbA1c: 7.1 ± 0.8%] were included in the analysis. They fasted overnight and then received a 0.2 U/kg subcutaneous dose of glulisine or aspart 2 min before starting a standardized test meal, 7 days apart, according to a randomization schedule. Blood samples were taken every 15 min, starting 20 min before the meal and ending 6 h postprandially.
Results: The area under the absolute glucose concentration-time curve between 0 and 1 h after insulin injection and maximal glucose concentration was significantly lower with glulisine than with aspart (p = 0.0455 and 0.0337, respectively). However, for the total study period, plasma glucose concentration was similar for glulisine and aspart. Peak insulin concentration was significantly higher for glulisine than for insulin aspart (p < 0.0001). Hypoglycaemic events (≤ 70 mg/dl with or without symptoms) occurred in 13 and 16 subjects treated with glulisine and aspart, respectively, but there were no cases of severe hypoglycaemia requiring intervention.
Conclusions: Glulisine was associated with lower glucose levels during the first hour after a standard meal; the remaining glucose profiles were otherwise equivalent, with higher insulin levels observed throughout the study period.
© 2011 Blackwell Publishing Ltd.
Figures

References
-
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. - PMC - PubMed
-
- Akalin S, Berntorp K, Ceriello A, et al. Intensive glucose therapy and clinical implications of recent data: a consensus statement from the Global Task Force on Glycaemic Control. Int J Clin Pract. 2009;63:1421–1425. - PubMed
-
- Albarrak AI, Luzio SD, Chassin LJ, Playle RA, Owens DR, Hovorka R. Associations of glucose control with insulin sensitivity and pancreatic beta-cell responsiveness in newly presenting type 2 diabetes. J Clin Endocrinol Metab. 2002;87:198–203. - PubMed
-
- Heinemann L, Chantelau EA, Starke AA. Pharmacokinetics and pharmacodynamics of subcutaneously administered U40 and U100 formulations of regular human insulin. Diabete Metab. 1992;18:21–24. - PubMed
-
- Becker RH. Insulin glulisine complementing basal insulins: a review of structure and activity. Diabetes Technol Ther. 2007;9:109–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

