Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes
- PMID: 21205128
- PMCID: PMC3084519
- DOI: 10.1111/j.1463-1326.2010.01356.x
Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes
Abstract
Aims: Most treatments for type 2 diabetes fail over time, necessitating combination therapy. We investigated the safety, tolerability and efficacy of liraglutide monotherapy compared with glimepiride monotherapy over 2 years.
Methods: Participants were randomized to receive once-daily liraglutide 1.2 mg, liraglutide 1.8 mg or glimepiride 8 mg. Participants completing the 1-year randomized, double-blind, double-dummy period could continue open-label treatment for an additional year. Safety data were evaluated for the full population exposed to treatment, and efficacy data were evaluated for the full intention-to-treat (ITT) and 2-year completer populations. Outcome measures included change in glycosylated haemoglobin (HbA1c), fasting plasma glucose (FPG), body weight and frequency of nausea and hypoglycaemia.
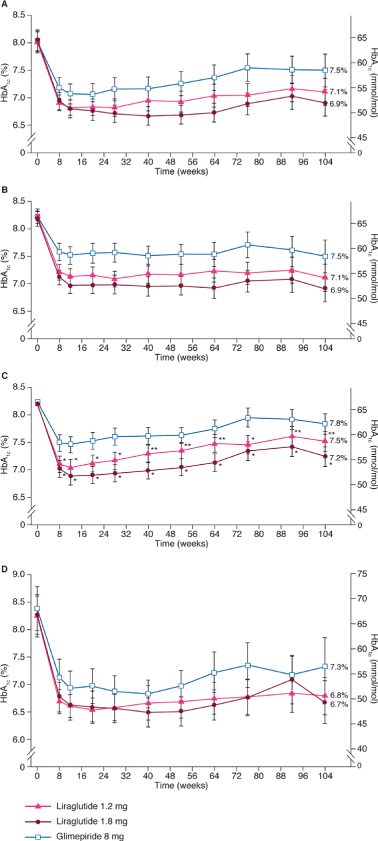
Results: For patients completing 2 years of therapy, HbA1c reductions were -0.6% with glimepiride versus -0.9% with liraglutide 1.2 mg (difference: -0.37, 95% CI: -0.71 to -0.02; p = 0.0376) and -1.1% with liraglutide 1.8 mg (difference: -0.55, 95% CI: -0.88 to -0.21; p = 0.0016). In the ITT population, HbA1c reductions were -0.3% with glimepiride versus -0.6% with liraglutide 1.2 mg (difference: -0.31, 95% CI: -0.54 to -0.08; p = 0.0076) and -0.9% with liraglutide 1.8 mg (difference: -0.60, 95% CI: -0.83 to -0.38; p < 0.0001). For both ITT and completer populations, liraglutide was more effective in reducing HbA1c, FPG and weight. Over 2 years, rates of minor hypoglycaemia [self-treated plasma glucose <3.1 mmol/l (<56 mg/dl)] were significantly lower with liraglutide 1.2 mg and 1.8 mg compared with glimepiride (p < 0.0001).
Conclusion: Liraglutide monotherapy for 2 years provides significant and sustained improvements in glycaemic control and body weight compared with glimepiride monotherapy, at a lower risk of hypoglycaemia.
© 2011 Blackwell Publishing Ltd.
Figures


References
-
- UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. - PubMed
-
- Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. - PubMed
-
- Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39–47. - PubMed
-
- Pratley RE, Nauck M, Bailey T, et al. 1860-LIRA-DPP-4 Study Group Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–1456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

