Coordinated activation of cellulose and repression of lignin biosynthesis pathways in rice
- PMID: 21205614
- PMCID: PMC3032476
- DOI: 10.1104/pp.110.168641
Coordinated activation of cellulose and repression of lignin biosynthesis pathways in rice
Abstract
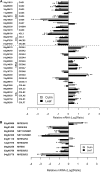
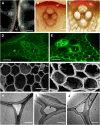
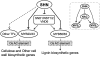
Cellulose from plant biomass is the largest renewable energy resource of carbon fixed from the atmosphere, which can be converted into fermentable sugars for production into ethanol. However, the cellulose present as lignocellulosic biomass is embedded in a hemicellulose and lignin matrix from which it needs to be extracted for efficient processing. Here, we show that expression of an Arabidopsis (Arabidopsis thaliana) transcription factor, SHINE (SHN), in rice (Oryza sativa), a model for the grasses, causes a 34% increase in cellulose and a 45% reduction in lignin content. The rice AtSHN lines also exhibit an altered lignin composition correlated with improved digestibility, with no compromise in plant strength and performance. Using a detailed systems-level analysis of global gene expression in rice, we reveal the SHN regulatory network coordinating down-regulation of lignin biosynthesis and up-regulation of cellulose and other cell wall biosynthesis pathway genes. The results thus support the development of nonfood crops and crop wastes with increased cellulose and low lignin with good agronomic performance that could improve the economic viability of lignocellulosic crop utilization for biofuels.
Figures

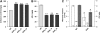


References
-
- Adkins NL, Hagerman TA, Georgel P. (2006) GAGA protein: a multi-faceted transcription factor. Biochem Cell Biol 84: 559–567 - PubMed
-
- Albersheim P, Nevins DJ, English PD, Karr A. (1967) A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr Res 5: 340–345
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 - PubMed
-
- Ambavaram MM, Pereira A. (2010) Setting up reverse transcription quantitative-PCR experiments. Methods Mol Biol 678: 45–54 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

