Evolutionary crossroads in developmental biology: Dictyostelium discoideum
- PMID: 21205784
- PMCID: PMC3014629
- DOI: 10.1242/dev.048934
Evolutionary crossroads in developmental biology: Dictyostelium discoideum
Abstract
Dictyostelium discoideum belongs to a group of multicellular life forms that can also exist for long periods as single cells. This ability to shift between uni- and multicellularity makes the group ideal for studying the genetic changes that occurred at the crossroads between uni- and multicellular life. In this Primer, I discuss the mechanisms that control multicellular development in Dictyostelium discoideum and reconstruct how some of these mechanisms evolved from a stress response in the unicellular ancestor.
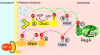
Figures





References
-
- Amagai A., Soramoto S. S., Saito S. H., Maeda Y. (2007). Ethylene induces zygote formation through an enhanced expression of zyg1 in Dictyostelium mucoroides. Exp. Cell Res. 313, 2493-2503 - PubMed
-
- Anjard C., Loomis W. F. (2006). GABA induces terminal differentiation of Dictyostelium through a GABA(B) receptor. Development 133, 2253-2261 - PubMed
-
- Anjard C., Loomis W. F. (2008). Cytokinins induce sporulation in Dictyostelium. Development 135, 819-827 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

