Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation
- PMID: 21205825
- PMCID: PMC3044954
- DOI: 10.1074/jbc.M110.166868
Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation
Abstract
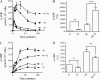
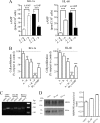
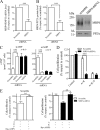
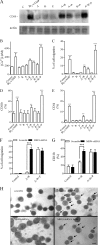
Increased intracellular cAMP concentration plays a well established role in leukemic cell maturation. We previously reported that U937 cells stimulated by H2 receptor agonists, despite a robust increase in cAMP, fail to mature because of rapid H2 receptor desensitization and phosphodiesterase (PDE) activation. Here we show that intracellular cAMP levels not only in U937 cells but also in other acute myeloid leukemia cell lines are also regulated by multidrug resistance-associated proteins (MRPs), particularly MRP4. U937, HL-60, and KG-1a cells, exposed to amthamine (H2-receptor agonist), augmented intracellular cAMP concentration with a concomitant increase in the efflux. Extrusion of cAMP was ATP-dependent and probenecid-sensitive, supporting that the transport was MRP-mediated. Cells exposed to amthamine and the PDE4 inhibitor showed enhanced cAMP extrusion, but this response was inhibited by MRP blockade. Amthamine stimulation, combined with PDE4 and MRP inhibition, induced maximal cell arrest proliferation. Knockdown strategy by shRNA revealed that this process was mediated by MRP4. Furthermore, blockade by probenecid or MRP4 knockdown showed that increased intracellular cAMP levels induce maturation in U937 cells. These findings confirm the key role of intracellular cAMP levels in leukemic cell maturation and provide the first evidence that MRP4 may represent a new potential target for leukemia differentiation therapy.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

