Genetics of type 1 diabetes
- PMID: 21205883
- PMCID: PMC4874193
- DOI: 10.1373/clinchem.2010.148221
Genetics of type 1 diabetes
Abstract
Background: Type 1 diabetes, a multifactorial disease with a strong genetic component, is caused by the autoimmune destruction of pancreatic β cells. The major susceptibility locus maps to the HLA class II genes at 6p21, although more than 40 non-HLA susceptibility gene markers have been confirmed.
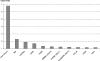
Content: Although HLA class II alleles account for up to 30%-50% of genetic type 1 diabetes risk, multiple non-MHC loci contribute to disease risk with smaller effects. These include the insulin, PTPN22, CTLA4, IL2RA, IFIH1, and other recently discovered loci. Genomewide association studies performed with high-density single-nucleotide-polymorphism genotyping platforms have provided evidence for a number of novel loci, although fine mapping and characterization of these new regions remain to be performed. Children born with the high-risk genotype HLADR3/4-DQ8 comprise almost 50% of children who develop antiislet autoimmunity by the age of 5 years. Genetic risk for type 1 diabetes can be further stratified by selection of children with susceptible genotypes at other diabetes genes, by selection of children with a multiple family history of diabetes, and/or by selection of relatives that are HLA identical to the proband.
Summary: Children with the HLA-risk genotypes DR3/4-DQ8 or DR4/DR4 who have a family history of type 1 diabetes have more than a 1 in 5 risk for developing islet autoantibodies during childhood, and children with the same HLA-risk genotype but no family history have approximately a 1 in 20 risk. Determining extreme genetic risk is a prerequisite for the implementation of primary prevention trials, which are now underway for relatives of individuals with type 1 diabetes.
Conflict of interest statement
Figures

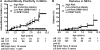


References
-
- Bell GI, Horita S, Karam JH. A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes. 1984;33:176–83. - PubMed
-
- Nistico L, Buzzetti R, Pritchard LE, VanderAuwera B, Giovannini C, Bosi E, et al. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Hum Mol Genet. 1996;5:1075–80. - PubMed
-
- Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

