T-cell receptor ligation induces distinct signaling pathways in naive vs. antigen-experienced T cells
- PMID: 21205892
- PMCID: PMC3029746
- DOI: 10.1073/pnas.1017340108
T-cell receptor ligation induces distinct signaling pathways in naive vs. antigen-experienced T cells
Abstract
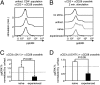
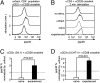
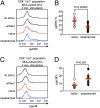
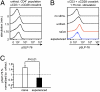
Naïve T lymphocytes display weaker and slower responses than antigen-experienced cells for reasons that are not well understood. Here we show that T-cell receptor (TCR) stimulation induces distinct ERK and p38 phosphorylation patterns in naïve and antigen-experienced human T cells, and that these contribute to the differential responses shown by these cells. Specifically, TCR ligation triggers the activation of the ERK pathway in naïve cells. This phosphorylation of ERK attenuates subsequent calcium influx and accelerates the degradation of the signalsome. In contrast, anti-CD3 stimulation of experienced cells results in the phosphorylation of p38 via an association with Discs large (Dlg). Thus, there are distinct signaling pathways triggered by TCR ligation that impair signaling in naïve cells and facilitate it in antigen-experienced cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wange RL. TCR signaling: another Abl-bodied kinase joins the cascade. Curr Biol. 2004;14:R562–R564. - PubMed
-
- van Oers NS. T cell receptor-mediated signs and signals governing T cell development. Semin Immunol. 1999;11:227–237. - PubMed
-
- Alberola-Ila J, Takaki S, Kerner JD, Perlmutter RM. Differential signaling by lymphocyte antigen receptors. Annu Rev Immunol. 1997;15:125–154. - PubMed
-
- Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

