Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice
- PMID: 21206087
- PMCID: PMC3026727
- DOI: 10.1172/JCI43984
Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice
Erratum in
-
Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice.J Clin Invest. 2016 Feb;126(2):795. doi: 10.1172/JCI85788. Epub 2016 Feb 1. J Clin Invest. 2016. PMID: 26829626 Free PMC article. No abstract available.
Abstract
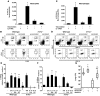
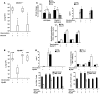
Worldwide rates of systemic fungal infections, including three of the major pathogens responsible for such infections in North America (Coccidioides posadasii, Histoplasma capsulatum, and Blastomyces dermatitidis), have soared recently, spurring interest in developing vaccines. The development of Th1 cells is believed to be crucial for protective immunity against pathogenic fungi, whereas the role of Th17 cells is vigorously debated. In models of primary fungal infection, some studies have shown that Th17 cells mediate resistance, while others have shown that they promote disease pathology. Here, we have shown that Th1 immunity is dispensable and that fungus-specific Th17 cells are sufficient for vaccine-induced protection against lethal pulmonary infection with B. dermatitidis in mice. Further, vaccine-induced Th17 cells were necessary and sufficient to protect against the three major systemic mycoses in North America. Mechanistically, Th17 cells engendered protection by recruiting and activating neutrophils and macrophages to the alveolar space, while the induction of Th17 cells and acquisition of vaccine immunity unexpectedly required the adapter molecule Myd88 but not the fungal pathogen recognition receptor Dectin-1. These data suggest that human vaccines against systemic fungal infections should be designed to induce Th17 cells if they are to be effective.
Figures
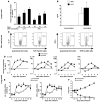
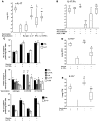

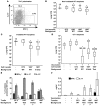
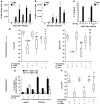
Comment in
-
Vaccines: Foes of fungi are just 17.Nat Rev Immunol. 2011 Feb;11(2):74. doi: 10.1038/nri2929. Nat Rev Immunol. 2011. PMID: 21467978 No abstract available.
Similar articles
-
C-type lectin receptors differentially induce th17 cells and vaccine immunity to the endemic mycosis of North America.J Immunol. 2014 Feb 1;192(3):1107-1119. doi: 10.4049/jimmunol.1302314. Epub 2014 Jan 3. J Immunol. 2014. PMID: 24391211 Free PMC article.
-
Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells.PLoS Pathog. 2012;8(7):e1002771. doi: 10.1371/journal.ppat.1002771. Epub 2012 Jul 19. PLoS Pathog. 2012. PMID: 22829762 Free PMC article.
-
The C-Type Lectin Receptor MCL Mediates Vaccine-Induced Immunity against Infection with Blastomyces dermatitidis.Infect Immun. 2015 Dec 14;84(3):635-42. doi: 10.1128/IAI.01263-15. Infect Immun. 2015. PMID: 26667836 Free PMC article.
-
The Role of the Interleukin-17 Axis and Neutrophils in the Pathogenesis of Endemic and Systemic Mycoses.Front Cell Infect Microbiol. 2020 Dec 14;10:595301. doi: 10.3389/fcimb.2020.595301. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33425780 Free PMC article. Review.
-
Pattern recognition: recent insights from Dectin-1.Curr Opin Immunol. 2009 Feb;21(1):30-7. doi: 10.1016/j.coi.2009.01.003. Epub 2009 Feb 14. Curr Opin Immunol. 2009. PMID: 19223162 Free PMC article. Review.
Cited by
-
An agonist of human complement fragment C5a enhances vaccine immunity against Coccidioides infection.Vaccine. 2012 Jun 29;30(31):4681-90. doi: 10.1016/j.vaccine.2012.04.084. Epub 2012 May 8. Vaccine. 2012. PMID: 22575167 Free PMC article.
-
Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses.Mucosal Immunol. 2017 Jan;10(1):260-270. doi: 10.1038/mi.2016.28. Epub 2016 Apr 6. Mucosal Immunol. 2017. PMID: 27049058
-
Th17 cell based vaccines in mucosal immunity.Curr Opin Immunol. 2013 Jun;25(3):373-80. doi: 10.1016/j.coi.2013.03.011. Epub 2013 May 10. Curr Opin Immunol. 2013. PMID: 23669353 Free PMC article. Review.
-
Interleukin 1 enhances vaccine-induced antifungal T-helper 17 cells and resistance against Blastomyces dermatitidis infection.J Infect Dis. 2013 Oct 1;208(7):1175-82. doi: 10.1093/infdis/jit283. Epub 2013 Jun 20. J Infect Dis. 2013. PMID: 23788728 Free PMC article.
-
Progress Toward a Human Vaccine Against Coccidioidomycosis.Curr Fungal Infect Rep. 2012 Dec 1;6(4):235-244. doi: 10.1007/s12281-012-0105-y. Curr Fungal Infect Rep. 2012. PMID: 23585916 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

