Role of the chemokine receptors CCR1, CCR2 and CCR4 in the pathogenesis of experimental dengue infection in mice
- PMID: 21206747
- PMCID: PMC3012079
- DOI: 10.1371/journal.pone.0015680
Role of the chemokine receptors CCR1, CCR2 and CCR4 in the pathogenesis of experimental dengue infection in mice
Abstract
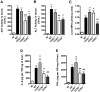
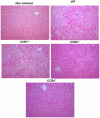
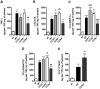
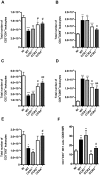
Dengue virus (DENV), a mosquito-borne flavivirus, is a public health problem in many tropical countries. Recent clinical data have shown an association between levels of different chemokines in plasma and severity of dengue. We evaluated the role of CC chemokine receptors CCR1, CCR2 and CCR4 in an experimental model of DENV-2 infection in mice. Infection of mice induced evident clinical disease and tissue damage, including thrombocytopenia, hemoconcentration, lymphopenia, increased levels of transaminases and pro-inflammatory cytokines, and lethality in WT mice. Importantly, infected WT mice presented increased levels of chemokines CCL2/JE, CCL3/MIP-1α and CCL5/RANTES in spleen and liver. CCR1⁻/⁻ mice had a mild phenotype with disease presentation and lethality similar to those of WT mice. In CCR2⁻/⁻ mice, lethality, liver damage, levels of IL-6 and IFN-γ, and leukocyte activation were attenuated. However, thrombocytopenia, hemoconcentration and systemic TNF-α levels were similar to infected WT mice. Infection enhanced levels of CCL17/TARC, a CCR4 ligand. In CCR4⁻/⁻ mice, lethality, tissue injury and systemic inflammation were markedly decreased. Despite differences in disease presentation in CCR-deficient mice, there was no significant difference in viral load. In conclusion, activation of chemokine receptors has discrete roles in the pathogenesis of dengue infection. These studies suggest that the chemokine storm that follows severe primary dengue infection associates mostly to development of disease rather than protection.
Conflict of interest statement
Figures
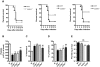
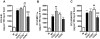


References
-
- Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. - PubMed
-
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–109. - PubMed
-
- Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, et al. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–321. - PubMed
-
- Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, et al. The WHO dengue classification and case definitions: time for a reassessment. Lancet. 2006;368:170–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

