A phase II trial of sorafenib in metastatic melanoma with tissue correlates
- PMID: 21206909
- PMCID: PMC3012061
- DOI: 10.1371/journal.pone.0015588
A phase II trial of sorafenib in metastatic melanoma with tissue correlates
Abstract
Background: Sorafenib monotherapy in patients with metastatic melanoma was explored in this multi-institutional phase II study. In correlative studies the impact of sorafenib on cyclin D1 and Ki67 was assessed.
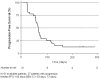
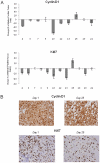
Methodology/principal findings: Thirty-six patients treatment-naïve advanced melanoma patients received sorafenib 400 mg p.o. twice daily continuously. Tumor BRAF(V600E) mutational status was determined by routine DNA sequencing and mutation-specific PCR (MSPCR). Immunohistochemistry (IHC) staining for cyclin D1 and Ki67 was performed on available pre- and post treatment tumor samples. The main toxicities included diarrhea, alopecia, rash, mucositis, nausea, hand-foot syndrome, and intestinal perforation. One patient had a RECIST partial response (PR) lasting 175 days. Three patients experienced stable disease (SD) with a mean duration of 37 weeks. Routine BRAF(V600E) sequencing yielded 27 wild-type (wt) and 6 mutant tumors, whereas MSPCR identified 12 wt and 18 mutant tumors. No correlation was seen between BRAF(V600E) mutational status and clinical activity. No significant changes in expression of cyclin D1 or Ki67 with sorafenib treatment were demonstrable in the 15 patients with pre-and post-treatment tumor samples.
Conclusions/significance: Sorafenib monotherapy has limited activity in advanced melanoma patients. BRAF(V600E) mutational status of the tumor was not associated with clinical activity and no significant effect of sorafenib on cyclin D1 or Ki67 was seen, suggesting that sorafenib is not an effective BRAF inhibitor or that additional signaling pathways are equally important in the patients who benefit from sorafenib.
Trial registration: Clinical Trials.gov NCT00119249.
Conflict of interest statement
Figures
References
-
- Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745. - PubMed
-
- Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745–2751. - PubMed
-
- Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. - PubMed
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
-
- Dong J, Phelps RG, Qiao R, Yao S, Benard O, et al. BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. Cancer Res. 2003;63:3883–3885. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

