Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes
- PMID: 21207025
- PMCID: PMC3426276
- DOI: 10.1007/s00262-010-0961-7
Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes
Abstract
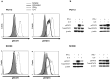
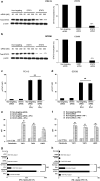
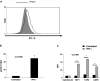
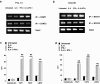
Squamous cell carcinoma of the head and neck (SCCHN) cells can escape recognition by tumor antigen (TA)-specific cytotoxic T lymphocytes (CTL) by downregulation of antigen processing machinery (APM) components, such as the transporter associated with antigen processing (TAP)-1/2 heterodimer. APM component upregulation by interferon gamma (IFN-γ) restores SCCHN cell recognition and susceptibility to lysis by CTL, but the mechanism underlying TAP1/2 downregulation in SCCHN cells is not known. Because IFN-γ activates signal transducer and activator of transcription (STAT)-1, we investigated phosphorylated (p)-STAT1 as a mediator of low basal TAP1/2 expression in SCCHN cells. SCCHN cells were found to express basal total STAT1 but low to undetectable levels of activated STAT1. The association of increased pSTAT1 levels and APM components likely reflects a cause-effect relationship, since STAT1 knockdown significantly reduced both IFN-γ-mediated APM component expression and TA-specific CTL recognition of IFN-γ-treated SCCHN cells. On the other hand, since oncogenic pSTAT3 is overexpressed in SCCHN cells and was found to heterodimerize with pSTAT1, we also tested whether pSTAT3 and pSTAT1:pSTAT3 heterodimers inhibited IFN-γ-induced STAT1 activation and APM component expression. First, STAT3 activation or depletion did not affect basal or IFN-γ-induced expression of pSTAT1 and APM components or recognition of SCCHN cells by TA-specific CTL. Second, pSTAT1:pSTAT3 heterodimers did not interfere with IFN-γ-induced STAT1 binding to the TAP1 promoter or APM protein expression. These findings demonstrate that APM component downregulation is regulated primarily by an IFN-γ-pSTAT1-mediated signaling pathway, independent of oncogenic STAT3 overexpression in SCCHN cells.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

