Early effects of orthodontic forces on osteoblast differentiation in a novel mouse organ culture model
- PMID: 21208081
- PMCID: PMC8925264
- DOI: 10.2319/052410-279.1
Early effects of orthodontic forces on osteoblast differentiation in a novel mouse organ culture model
Abstract
Objective: To develop a mouse orthodontic organ culture model and examine early-induced changes in osteoblast differentiation markers within the periodontal ligament (PDL) and alveolar bone.
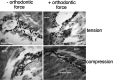
Methods: Mandibles from 4- to 12-week-old transgenic mice were dissected and hemisected. A conventional superelastic orthodontic spring (25 grams) was bonded to the incisor and first molar on one side of the mandible; the other side served as a control. Dissected mandibles were cultured for 6 hours and then were histologically analyzed for proliferation (BrdU immunostaining) and fluorescent protein expression. Additionally, an in vivo model using the same methods was applied to 3.6 Col1-GFP transgenic mice.
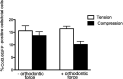
Results: In vitro, after 6 hours of orthodontic loading, a significant increase was noted in 3.6Col1-GFP- and BSP-GFP-positive cells within the tension side of the PDL compared with unloaded controls. On the compression side, a significant decrease in positive cells in 3.6Col1-GFP mice was observed in the PDL compared with unloaded controls. In vivo, the same tendencies were found.
Conclusion: This novel in vitro mandibular tooth movement organ culture model coupled with transgenic mouse technology provides a powerful tool for delineating initial cellular and molecular events of orthodontic tooth movement.
Figures






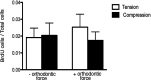


Similar articles
-
Orthodontic tooth movement causes decreased promoter expression of collagen type 1, bone sialoprotein and alpha-smooth muscle actin in the periodontal ligament.Orthod Craniofac Res. 2012 Feb;15(1):52-61. doi: 10.1111/j.1601-6343.2011.01536.x. Orthod Craniofac Res. 2012. PMID: 22264327
-
Evaluation of BSP expression and apoptosis in the periodontal ligament during orthodontic relapse: a preliminary study.Orthod Craniofac Res. 2014 Nov;17(4):239-48. doi: 10.1111/ocr.12049. Epub 2014 Jun 13. Orthod Craniofac Res. 2014. PMID: 24924469
-
Effect of mechanical loading on periodontal cells.Crit Rev Oral Biol Med. 2001;12(5):414-24. doi: 10.1177/10454411010120050401. Crit Rev Oral Biol Med. 2001. PMID: 12002823 Review.
-
Temporal and spatial mRNA expression of bone sialoprotein and type I collagen during rodent tooth movement.Eur J Orthod. 2001 Aug;23(4):339-48. doi: 10.1093/ejo/23.4.339. Eur J Orthod. 2001. PMID: 11544783
-
Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement.Front Oral Biol. 2016;18:1-8. doi: 10.1159/000351894. Epub 2015 Nov 24. Front Oral Biol. 2016. PMID: 26599112 Free PMC article. Review.
Cited by
-
Biomarkers of periodontal tissue remodeling during orthodontic tooth movement in mice and men: overview and clinical relevance.ScientificWorldJournal. 2013 Apr 23;2013:105873. doi: 10.1155/2013/105873. Print 2013. ScientificWorldJournal. 2013. PMID: 23737704 Free PMC article. Review.
-
Osteoimmunology in Periodontitis and Orthodontic Tooth Movement.Curr Osteoporos Rep. 2023 Apr;21(2):128-146. doi: 10.1007/s11914-023-00774-x. Epub 2023 Mar 2. Curr Osteoporos Rep. 2023. PMID: 36862360 Free PMC article. Review.
-
Impacts of Glucose-Dependent Insulinotropic Polypeptide on Orthodontic Tooth Movement-Induced Bone Remodeling.Int J Mol Sci. 2022 Aug 10;23(16):8922. doi: 10.3390/ijms23168922. Int J Mol Sci. 2022. PMID: 36012183 Free PMC article.
-
Mechanistic Insight into Orthodontic Tooth Movement Based on Animal Studies: A Critical Review.J Clin Med. 2021 Apr 16;10(8):1733. doi: 10.3390/jcm10081733. J Clin Med. 2021. PMID: 33923725 Free PMC article. Review.
-
Dental Anomalies Associated with Craniometaphyseal Dysplasia.J Dent Res. 2014 Jun;93(6):553-8. doi: 10.1177/0022034514529304. Epub 2014 Mar 24. J Dent Res. 2014. PMID: 24663682 Free PMC article.
References
-
- Storey E. The nature of tooth movement. Am J Orthod. 1973;63:292–314. - PubMed
-
- Hosoya A, Ninomiya T, Hiraga T, et al. Alveolar bone regeneration of subcutaneously transplanted rat molar. Bone. 2008;42:350–357. - PubMed
-
- Mukai M, Yoshimine Y, Akamine A, Maeda K. Bone-like nodules formed in vitro by rat periodontal ligament cells. Cell Tissue Res. 1993;271:453–460. - PubMed
-
- Aubin J. E, Liu F, Malaval L, Gupta A. K. Osteoblast and chondroblast differentiation. Bone. 1995;17:77S–83S. - PubMed
-
- Dacic S, Kalajzic I, Visnjic D, Lichtler A. C, Rowe D. W. Col1a1-driven transgenic markers of osteoblast lineage progression. J Bone Miner Res. 2001;16:1228–1236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

