Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial
- PMID: 21208974
- PMCID: PMC3069389
- DOI: 10.1093/eurheartj/ehq502
Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial
Abstract
Aims: To evaluate efficacy and safety of RLY5016 (a non-absorbed, orally administered, potassium [K+]-binding polymer) on serum K+ levels in patients with chronic heart failure (HF) receiving standard therapy and spironolactone.
Methods and results: One hundred and five patients with HF and a history of hyperkalaemia resulting in discontinuation of a renin-angiotensin-aldosterone system inhibitor/blocker and/or beta-adrenergic blocking agent or chronic kidney disease (CKD) with an estimated glomerular filtration rate of <60 mL/min were randomized to double-blind treatment with 30 g/day RLY5016 or placebo for 4 weeks. Spironolactone, initiated at 25 mg/day, was increased to 50 mg/day on Day 15 if K+ was ≤5.1 mEq/L. Endpoints included the change from baseline in serum K+ at the end of treatment (primary); the proportion of patients with hyperkalaemia (K+ >5.5 mEq/L); and the proportion titrated to spironolactone 50 mg/day. Safety assessments included adverse events (AEs) and clinical laboratory tests. RLY5016 (n= 55) and placebo (n= 49) patients had similar baseline characteristics. At the end of treatment, compared with placebo, RLY5016 had significantly lowered serum K+ levels with a difference between groups of -0.45 mEq/L (P < 0.001); a lower incidence of hyperkalaemia (7.3% RLY5016 vs. 24.5% placebo, P= 0.015); and a higher proportion of patients on spironolactone 50 mg/day (91% RLY5016 vs. 74% placebo, P= 0.019). In patients with CKD (n= 66), the difference in K+ between groups was -0.52 mEq/L (P= 0.031), and the incidence of hyperkalaemia was 6.7% RLY5016 vs. 38.5% placebo (P= 0.041). Adverse events were mainly gastrointestinal, and mild or moderate in severity. Adverse events resulting in study withdrawal were similar (7% RLY5016, 6% placebo). There were no drug-related serious AEs. Hypokalaemia (K+ <3.5 mEq/L) occurred in 6% of RLY5016 patients vs. 0% of placebo patients (P= 0.094).
Conclusion: RLY5016 prevented hyperkalaemia and was relatively well tolerated in patients with HF receiving standard therapy and spironolactone (25-50 mg/day).
Trial registration: ClinicalTrials.gov NCT00868439.
Figures
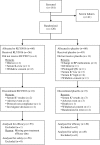
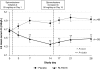
Comment in
-
To bind or not to bind: potassium-lowering drugs in heart failure.Eur Heart J. 2011 Apr;32(7):791-2. doi: 10.1093/eurheartj/ehr058. Epub 2011 Mar 7. Eur Heart J. 2011. PMID: 21383002 No abstract available.
References
-
- Pitt B. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi:10.1056/NEJM199909023411001. - DOI - PubMed
-
- Zannad F, McMurray JJV, Drexler H, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pitt B. Rationale and design of the Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure (EMPHASIS-HF) Eur J Heart Fail. 2010;12:617–622. - PubMed
-
- Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70:2116–2123. - PubMed
-
- Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1:940–951. doi:10.2215/CJN.00240106. - DOI - PubMed
-
- Palmer BF. Hypertension management in patients with chronic kidney disease. Curr Hypertens Rep. 2008;10:367–373. doi:10.1007/s11906-008-0069-z. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

