A compact beta model of huntingtin toxicity
- PMID: 21209075
- PMCID: PMC3048705
- DOI: 10.1074/jbc.M110.192013
A compact beta model of huntingtin toxicity
Abstract
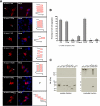
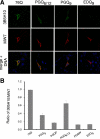
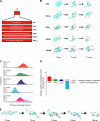
Huntington disease results from an expanded polyglutamine region in the N terminus of the huntingtin protein. HD pathology is characterized by neuronal degeneration and protein inclusions containing N-terminal fragments of mutant huntingtin. Structural information is minimal, though it is believed that mutant huntingtin polyglutamine adopts β structure upon conversion to a toxic form. To this end, we designed mammalian cell expression constructs encoding compact β variants of Htt exon 1 N-terminal fragment and tested their ability to aggregate and induce toxicity in cultured neuronal cells. In parallel, we performed molecular dynamics simulations, which indicate that constructs with expanded polyglutamine β-strands are stabilized by main-chain hydrogen bonding. Finally, we found a correlation between the reactivity to 3B5H10, an expanded polyglutamine antibody that recognizes a compact β rich hairpin structure, and the ability to induce cell toxicity. These data are consistent with an important role for a compact β structure in mutant huntingtin-induced cell toxicity.
Figures

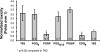
References
-
- Ross C. A., Margolis R. L., Rosenblatt A., Ranen N. G., Becher M. W., Aylward E. (1997) Medicine 76, 305–338 - PubMed
-
- Zoghbi H. Y., Orr H. T. (2000) Annu. Rev. Neurosci. 23, 217–247 - PubMed
-
- Huntington's Disease Collaborative Research Group (1993) Cell 72, 971–983 - PubMed
-
- Ross C. A., Tabrizi S. J. (2011) Lancet 10, 83–98 - PubMed
-
- Andrade M. A., Bork P. (1995) Nat. Genet. 11, 115–116 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

