S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release
- PMID: 21209080
- PMCID: PMC3044978
- DOI: 10.1074/jbc.M110.169342
S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release
Abstract
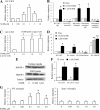
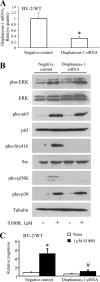
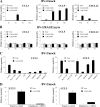
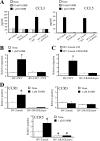
The Ca(2+)-binding protein of the EF-hand type, S100B, is abundantly expressed in and secreted by astrocytes, and release of S100B from damaged astrocytes occurs during the course of acute and chronic brain disorders. Thus, the concept has emerged that S100B might act an unconventional cytokine or a damage-associated molecular pattern protein playing a role in the pathophysiology of neurodegenerative disorders and inflammatory brain diseases. S100B proinflammatory effects require relatively high concentrations of the protein, whereas at physiological concentrations S100B exerts trophic effects on neurons. Most if not all of the extracellular (trophic and toxic) effects of S100B in the brain are mediated by the engagement of RAGE (receptor for advanced glycation end products). We show here that high S100B stimulates murine microglia migration in Boyden chambers via RAGE-dependent activation of Src kinase, Ras, PI3K, MEK/ERK1/2, RhoA/ROCK, Rac1/JNK/AP-1, Rac1/NF-κB, and, to a lesser extent, p38 MAPK. Recruitment of the adaptor protein, diaphanous-1, a member of the formin protein family, is also required for S100B/RAGE-induced migration of microglia. The S100B/RAGE-dependent activation of diaphanous-1/Rac1/JNK/AP-1, Ras/Rac1/NF-κB and Src/Ras/PI3K/RhoA/diaphanous-1 results in the up-regulation of expression of the chemokines, CCL3, CCL5, and CXCL12, whose release and activity are required for S100B to stimulate microglia migration. Lastly, RAGE engagement by S100B in microglia results in up-regulation of the chemokine receptors, CCR1 and CCR5. These results suggests that S100B might participate in the pathophysiology of brain inflammatory disorders via RAGE-dependent regulation of several inflammation-related events including activation and migration of microglia.
Figures

References
-
- Donato R., Sorci G., Riuzzi F., Arcuri C., Bianchi R., Brozzi F., Tubaro C., Giambanco I. (2009) Biochim. Biophys. Acta 1793, 1008–1022 - PubMed
-
- Sorci G., Agneletti A. L., Donato R. (2000) Neuroscience 99, 773–783 - PubMed
-
- Xiong Z., O'Hanlon D., Becker L. E., Roder J., MacDonald J. F., Marks A. (2000) Exp. Cell Res. 257, 281–289 - PubMed
-
- Van Eldik L. J., Wainwright M. S. (2003) Restor. Neurol. Neurosci. 21, 97–108 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

