The exon junction complex component Y14 modulates the activity of the methylosome in biogenesis of spliceosomal small nuclear ribonucleoproteins
- PMID: 21209085
- PMCID: PMC3058967
- DOI: 10.1074/jbc.M110.190587
The exon junction complex component Y14 modulates the activity of the methylosome in biogenesis of spliceosomal small nuclear ribonucleoproteins
Abstract
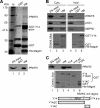
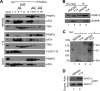
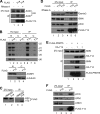
The RNA-binding protein Y14 heterodimerizes with Mago as the core of the exon junction complex during precursor mRNA splicing and plays a role in mRNA surveillance in the cytoplasm. Using the Y14/Magoh heterodimer as bait in a screening for its interacting partners, we identified the protein-arginine methyltransferase PRMT5 as a candidate. We show that Y14 and Magoh, but not other factors of the exon junction complex, interact with the cytoplasmic PRMT5-containing methylosome. We further provide evidence that Y14 promoted the activity of PRMT5 in methylation of Sm proteins of the small nuclear ribonucleoprotein core, whereas knockdown of Y14 reduced their methylation level. Moreover, Y14 overexpression induced the formation of a large, active, and small nuclear ribonucleoprotein (snRNP)-associated methylosome complex. However, Y14 may only transiently associate with the snRNP assembly complex in the cytoplasm. Together, our results suggest that Y14 facilitates Sm protein methylation probably by its activity in promoting the formation or stability of the methylosome-containing complex. We hypothesize that Y14 provides a regulatory link between pre-mRNA splicing and snRNP biogenesis.
Figures



Similar articles
-
RioK1, a new interactor of protein arginine methyltransferase 5 (PRMT5), competes with pICln for binding and modulates PRMT5 complex composition and substrate specificity.J Biol Chem. 2011 Jan 21;286(3):1976-86. doi: 10.1074/jbc.M110.148486. Epub 2010 Nov 16. J Biol Chem. 2011. PMID: 21081503 Free PMC article.
-
A novel WD repeat protein component of the methylosome binds Sm proteins.J Biol Chem. 2002 Mar 8;277(10):8243-7. doi: 10.1074/jbc.M109984200. Epub 2001 Dec 26. J Biol Chem. 2002. PMID: 11756452
-
Structure of the Y14-Magoh core of the exon junction complex.Curr Biol. 2003 May 27;13(11):933-41. doi: 10.1016/s0960-9822(03)00328-2. Curr Biol. 2003. PMID: 12781131
-
Deciphering the assembly pathway of Sm-class U snRNPs.FEBS Lett. 2008 Jun 18;582(14):1997-2003. doi: 10.1016/j.febslet.2008.03.009. Epub 2008 Mar 17. FEBS Lett. 2008. PMID: 18348870 Review.
-
The SMN complex: an assembly machine for RNPs.Cold Spring Harb Symp Quant Biol. 2006;71:313-20. doi: 10.1101/sqb.2006.71.001. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381311 Review.
Cited by
-
Localized Inhibition of Protein Phosphatase 1 by NUAK1 Promotes Spliceosome Activity and Reveals a MYC-Sensitive Feedback Control of Transcription.Mol Cell. 2020 Mar 19;77(6):1322-1339.e11. doi: 10.1016/j.molcel.2020.01.008. Epub 2020 Jan 31. Mol Cell. 2020. PMID: 32006464 Free PMC article.
-
The RNA-binding protein Y14 inhibits mRNA decapping and modulates processing body formation.Mol Biol Cell. 2013 Jan;24(1):1-13. doi: 10.1091/mbc.E12-03-0217. Epub 2012 Oct 31. Mol Biol Cell. 2013. PMID: 23115303 Free PMC article.
-
Exon junction complex (EJC) core genes play multiple developmental roles in Physalis floridana.Plant Mol Biol. 2018 Dec;98(6):545-563. doi: 10.1007/s11103-018-0795-9. Epub 2018 Nov 13. Plant Mol Biol. 2018. PMID: 30426309 Free PMC article.
-
PRMT5 is upregulated in malignant and metastatic melanoma and regulates expression of MITF and p27(Kip1.).PLoS One. 2013 Sep 30;8(9):e74710. doi: 10.1371/journal.pone.0074710. eCollection 2013. PLoS One. 2013. PMID: 24098663 Free PMC article.
-
Phosphorylation status of human RNA-binding protein 8A in cells and its inhibitory regulation by Magoh.Exp Biol Med (Maywood). 2015 Apr;240(4):438-45. doi: 10.1177/1535370214556945. Epub 2014 Oct 27. Exp Biol Med (Maywood). 2015. PMID: 25349214 Free PMC article.
References
-
- Gehring N. H., Lamprinaki S., Kulozik A. E., Hentze M. W. (2009) Cell 137, 536–548 - PubMed
-
- Degot S., Le Hir H., Alpy F., Kedinger V., Stoll I., Wendling C., Seraphin B., Rio M. C., Tomasetto C. (2004) J. Biol. Chem. 279, 33702–33715 - PubMed
-
- Palacios I. M., Gatfield D., St Johnston D., Izaurralde E. (2004) Nature 427, 753–757 - PubMed
-
- Gehring N. H., Neu-Yilik G., Schell T., Hentze M. W., Kulozik A. E. (2003) Mol. Cell 11, 939–949 - PubMed
-
- Fribourg S., Gatfield D., Izaurralde E., Conti E. (2003) Nat. Struct. Biol. 10, 433–439 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

