Metabolic fate of fumarate, a side product of the purine salvage pathway in the intraerythrocytic stages of Plasmodium falciparum
- PMID: 21209090
- PMCID: PMC3059058
- DOI: 10.1074/jbc.M110.173328
Metabolic fate of fumarate, a side product of the purine salvage pathway in the intraerythrocytic stages of Plasmodium falciparum
Abstract
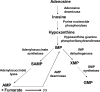
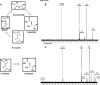
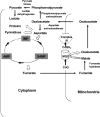
In aerobic respiration, the tricarboxylic acid cycle is pivotal to the complete oxidation of carbohydrates, proteins, and lipids to carbon dioxide and water. Plasmodium falciparum, the causative agent of human malaria, lacks a conventional tricarboxylic acid cycle and depends exclusively on glycolysis for ATP production. However, all of the constituent enzymes of the tricarboxylic acid cycle are annotated in the genome of P. falciparum, which implies that the pathway might have important, yet unidentified biosynthetic functions. Here we show that fumarate, a side product of the purine salvage pathway and a metabolic intermediate of the tricarboxylic acid cycle, is not a metabolic waste but is converted to aspartate through malate and oxaloacetate. P. falciparum-infected erythrocytes and free parasites incorporated [2,3-(14)C]fumarate into the nucleic acid and protein fractions. (13)C NMR of parasites incubated with [2,3-(13)C]fumarate showed the formation of malate, pyruvate, lactate, and aspartate but not citrate or succinate. Further, treatment of free parasites with atovaquone inhibited the conversion of fumarate to aspartate, thereby indicating this pathway as an electron transport chain-dependent process. This study, therefore, provides a biosynthetic function for fumarate hydratase, malate quinone oxidoreductase, and aspartate aminotransferase of P. falciparum.
Figures





References
-
- Bryant C., Voller A., Smith M. J. (1964) Am. J. Trop. Med. Hyg. 13, 515–519 - PubMed
-
- Kirk K., Horner H. A., Kirk J. (1996) Mol. Biochem. Parasitol. 82, 195–205 - PubMed
-
- Foth B. J., Stimmler L. M., Handman E., Crabb B. S., Hodder A. N., McFadden G. I. (2005) Mol. Microbiol. 55, 39–53 - PubMed
-
- Chen J., Jones M. (1976) Arch. Biochem. Biophys. 176, 82–90 - PubMed
-
- Wrenger C., Muller S. (2003) Eur. J. Biochem. 270, 1775–1783 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

