Inhibition of beta-secretase in vivo via antibody binding to unique loops (D and F) of BACE1
- PMID: 21209097
- PMCID: PMC3048749
- DOI: 10.1074/jbc.M110.194860
Inhibition of beta-secretase in vivo via antibody binding to unique loops (D and F) of BACE1
Abstract
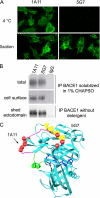
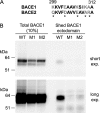
β-Secretase (BACE1) is an attractive drug target for Alzheimer disease. However, the design of clinical useful inhibitors targeting its active site has been extremely challenging. To identify alternative drug targeting sites we have generated a panel of BACE1 monoclonal antibodies (mAbs) that interfere with BACE1 activity in various assays and determined their binding epitopes. mAb 1A11 inhibited BACE1 in vitro using a large APP sequence based substrate (IC(50) ∼0.76 nm), in primary neurons (EC(50) ∼1.8 nm), and in mouse brain after stereotactic injection. Paradoxically, mAb 1A11 increased BACE1 activity in vitro when a short synthetic peptide was used as substrate, indicating that mAb 1A11 does not occupy the active-site. Epitope mapping revealed that mAb 1A11 binds to adjacent loops D and F, which together with nearby helix A, distinguishes BACE1 from other aspartyl proteases. Interestingly, mutagenesis of loop F and helix A decreased or increased BACE1 activity, identifying them as enzymatic regulatory elements and as potential alternative sites for inhibitor design. In contrast, mAb 5G7 was a potent BACE1 inhibitor in cell-free enzymatic assays (IC(50) ∼0.47 nm) but displayed no inhibitory effect in primary neurons. Its epitope, a surface helix 299-312, is inaccessible in membrane-anchored BACE1. Remarkably, mutagenesis of helix 299-312 strongly reduced BACE1 ectodomain shedding, suggesting that this helix plays a role in BACE1 cellular biology. In conclusion, this study generated highly selective and potent BACE1 inhibitory mAbs, which recognize unique structural and functional elements in BACE1, and uncovered interesting alternative sites on BACE1 that could become targets for drug development.
Figures






References
-
- Hardy J., Selkoe D. J. (2002) Science 297, 353–356 - PubMed
-
- Golde T. E., Dickson D., Hutton M. (2006) Curr. Alzheimer Res. 3, 421–430 - PubMed
-
- Selkoe D. J. (2001) Physiol. Rev. 81, 741–766 - PubMed
-
- Hussain I., Powell D., Howlett D. R., Tew D. G., Meek T. D., Chapman C., Gloger I. S., Murphy K. E., Southan C. D., Ryan D. M., Smith T. S., Simmons D. L., Walsh F. S., Dingwall C., Christie G. (1999) Mol. Cell Neurosci. 14, 419–427 - PubMed
-
- Sinha S., Anderson J. P., Barbour R., Basi G. S., Caccavello R., Davis D., Doan M., Dovey H. F., Frigon N., Hong J., Jacobson-Croak K., Jewett N., Keim P., Knops J., Lieberburg I., Power M., Tan H., Tatsuno G., Tung J., Schenk D., Seubert P., Suomensaari S. M., Wang S., Walker D., Zhao J., McConlogue L., John V. (1999) Nature 402, 537–540 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

