Subcellular localization and rearrangement of endoplasmic reticulum by Brome mosaic virus capsid protein
- PMID: 21209103
- PMCID: PMC3067956
- DOI: 10.1128/JVI.02020-10
Subcellular localization and rearrangement of endoplasmic reticulum by Brome mosaic virus capsid protein
Abstract
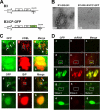
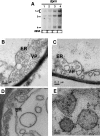
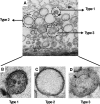
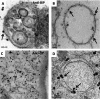
Genome packaging in the plant-infecting Brome mosaic virus (BMV), a member of the alphavirus-like superfamily, as well as in other positive-strand RNA viruses pathogenic to humans (e.g., poliovirus) and animals (e.g., Flock House virus), is functionally coupled to replication. Although the subcellular localization site of BMV replication has been identified, that of the capsid protein (CP) has remained elusive. In this study, the application of immunofluorescence confocal microscopy to Nicotiana benthamiana leaves expressing replication-derived BMV CP as a green fluorescent protein (GFP) fusion, in conjunction with antibodies to the CP and double-stranded RNA, a presumed marker of RNA replication, revealed that the subcellular localization sites of replication and CP overlap. Our temporal analysis by transmission electron microscopy of ultrastructural modifications induced in BMV-infected N. benthamiana leaves revealed a reticulovesicular network of modified endoplasmic reticulum (ER) incorporating large assemblies of vesicles derived from ER accumulated in the cytoplasm during BMV infection. Additionally, for the first time, we have found by ectopic expression experiments that BMV CP itself has the intrinsic property of modifying ER to induce vesicles similar to those present in BMV infections. The significance of CP-induced vesicles in relation to CP-organized viral functions that are linked to replication-coupled packaging is discussed.
Figures




References
-
- Annamalai, P., and A. L. Rao. 2006. Delivery and expression of functional viral RNA genomes in planta by agroinfiltration, unit 16B, p. 2.1-2.15. In T. Downey (ed.), Current protocols in microbiology. John Wiley & Sons Inc., Mississauga, Ontario, Canada. - PubMed
-
- Annamalai, P., and A. L. Rao. 2005. Replication-independent expression of genome components and capsid protein of brome mosaic virus in planta: a functional role for viral replicase in RNA packaging. Virology 338:96-111. - PubMed
-
- Annamalai, P., F. Rofail, D. A. Demason, and A. L. Rao. 2008. Replication-coupled packaging mechanism in positive-strand RNA viruses: synchronized coexpression of functional multigenome RNA components of an animal and a plant virus in Nicotiana benthamiana cells by agroinfiltration. J. Virol. 82:1484-1495. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

