Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands
- PMID: 21209157
- PMCID: PMC3067385
- DOI: 10.1128/CVI.00396-10
Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands
Abstract
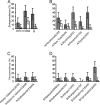
To gain insight into the age at which children become infected with influenza viruses for the first time, we analyzed the seroprevalence of antibodies against influenza viruses in children 0 to 7 years of age in the Netherlands. Serum samples were collected during a cross-sectional population-based study in 2006 and 2007 and were tested for the presence of antibodies against influenza A/H1N1, A/H3N2, and B viruses representative of viruses present in previous influenza seasons using the hemagglutination inhibition assay. The seroprevalence of antibodies to influenza virus was higher in children 1 to 6 months of age than in children 7 to 12 months of age, which likely reflects the presence of maternally derived antibodies. The proportion of study subjects >1 year of age with detectable antibodies against influenza viruses gradually increased with age until they reached the age of 6 years, when they all had antibodies to at least one influenza A virus. These findings may have implications for the development of vaccination strategies aiming at the protection of young children against seasonal and/or pandemic influenza virus infection.
Figures
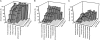


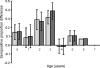
References
-
- Bodewes, R., J. H. Kreijtz, and G. F. Rimmelzwaan. 2009. Yearly influenza vaccinations: a double-edged sword? Lancet Infect. Dis. 9:784-788. - PubMed
-
- Dawood, F. S., et al. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605-2615. - PubMed
-
- De Jong, J. C., et al. 2003. Haemagglutination-inhibiting antibody to influenza virus. Dev. Biol. (Basel) 115:63-73. - PubMed
-
- de Jong, J. C., et al. 2005. The influenza season 2004/'05 in the Netherlands with the largest epidemic of the last 5 years caused by the virus variant A/California and the composition of the vaccine for the season 2005/'06. Ned. Tijdschr. Geneeskd. 149:2355-2361. - PubMed
-
- de Jong, J. C., et al. 2001. 2000/01 influenza season and the vaccine composition for the season 2001/'02. Ned. Tijdschr. Geneeskd. 145:1945-1950. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

