Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: implications for Parkinson's disease
- PMID: 21209200
- PMCID: PMC3039694
- DOI: 10.1523/JNEUROSCI.1833-10.2011
Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: implications for Parkinson's disease
Abstract
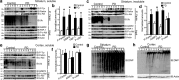
Mutations in parkin, an E3 ubiquitin ligase, are the most common cause of autosomal-recessive Parkinson's disease (PD). Here, we show that the stress-signaling non-receptor tyrosine kinase c-Abl links parkin to sporadic forms of PD via tyrosine phosphorylation. Under oxidative and dopaminergic stress, c-Abl was activated in cultured neuronal cells and in striatum of adult C57BL/6 mice. Activated c-Abl was found in the striatum of PD patients. Concomitantly, parkin was tyrosine-phosphorylated, causing loss of its ubiquitin ligase and cytoprotective activities, and the accumulation of parkin substrates, AIMP2 (aminoacyl tRNA synthetase complex-interacting multifunctional protein 2) (p38/JTV-1) and FBP-1.STI-571, a selective c-Abl inhibitor, prevented tyrosine phosphorylation of parkin and restored its E3 ligase activity and cytoprotective function both in vitro and in vivo. Our results suggest that tyrosine phosphorylation of parkin by c-Abl is a major post-translational modification that leads to loss of parkin function and disease progression in sporadic PD. Moreover, inhibition of c-Abl offers new therapeutic opportunities for blocking PD progression.
Figures



References
-
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. - PubMed
-
- Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, Schellens JH. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–2582. - PubMed
-
- Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med. 2001;7:1144–1150. - PubMed
-
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. - PubMed
-
- Corti O, Hampe C, Koutnikova H, Darios F, Jacquier S, Prigent A, Robinson JC, Pradier L, Ruberg M, Mirande M, Hirsch E, Rooney T, Fournier A, Brice A. The p38 subunit of the aminoacyl-tRNA synthetase complex is a Parkin substrate: linking protein biosynthesis and neurodegeneration. Hum Mol Genet. 2003;12:1427–1437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
