The neuronal basis of long-term sensorimotor learning
- PMID: 21209216
- PMCID: PMC6622741
- DOI: 10.1523/JNEUROSCI.4055-10.2011
The neuronal basis of long-term sensorimotor learning
Abstract
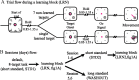
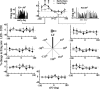
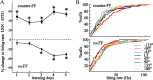
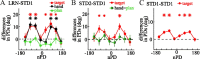
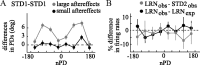
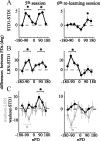
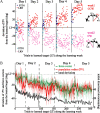
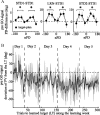
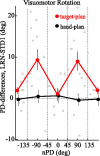
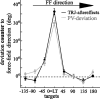
The brain has a remarkable ability to learn and adjust behavior. For instance, the brain can adjust muscle activation to cope with changes in the environment. However, the neuronal mechanisms behind this adaptation are not clear. To address this fundamental question, this study examines the neuronal basis of long-term sensorimotor learning by recording neuronal activity in the primary motor cortex of monkeys during a long-term adaptation to a force-field perturbation. For 5 consecutive days, the same perturbation was applied to the monkey's hand when reaching to a single target, whereas movements to all other targets were not perturbed. The gradual improvement in performance over these 5 days was correlated to the evolvement in the population neuronal signal, with two timescales of changes in single-cell activity. Specifically, one subgroup of cells showed a relatively fast increase in activity, whereas the other showed a gradual, slower decrease. These adapted patterns of neuronal activity did not involve changes in directional tuning of single cells, suggesting that adaptation was the result of adjustments of the required motor plan by a population of neurons rather than changes in single-cell properties. Furthermore, generalization was mostly expressed in the direction of the required compensatory force during adaptation. Altogether, the neuronal activity and its generalization accord with the adapted motor plan.
Figures


References
-
- Burdet E, Osu R, Franklin DW, Milner TE, Kawato M. The central nervous system stabilizes unstable dynamics by learning optimal impedance. Nature. 2001;414:446–449. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
