Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis
- PMID: 21209700
- PMCID: PMC3000428
- DOI: 10.1371/journal.pone.0015531
Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis
Abstract
Background: There is consensus that experimental autoimmune encephalomyelitis (EAE) can be mediated by myelin specific T cells of Th1 as well as of Th17 phenotype, but the contribution of either subset to the pathogenic process has remained controversial. In this report, we compare functional differences and pathogenic potential of "monoclonal" T cell lines that recognize myelin oligodendrocyte glycoprotein (MOG) with the same transgenic TCR but are distinguished by an IFN-γ producing Th1-like and IL-17 producing Th17-like cytokine signature.
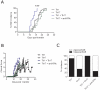
Methods and findings: CD4+ T cell lines were derived from the transgenic mouse strain 2D2, which expresses a TCR recognizing MOG peptide 35-55 in the context of I-A(b). Adoptive transfer of Th1 cells into lymphopenic (Rag2⁻/⁻) recipients, predominantly induced "classic" paralytic EAE, whereas Th17 cells mediated "atypical" ataxic EAE in approximately 50% of the recipient animals. Combination of Th1 and Th17 cells potentiated the encephalitogenicity inducing classical EAE exclusively. Th1 and Th17 mediated EAE lesions differed in their composition but not in their localization within the CNS. While Th1 lesions contained IFN-γ, but no IL-17 producing T cells, the T cells in Th17 lesions showed plasticity, substantially converting to IFN-γ producing Th1-like cells. Th1 and Th17 cells differed drastically by their lytic potential. Th1 but not Th17 cells lysed autoantigen presenting astrocytes and fibroblasts in vitro in a contact-dependent manner. In contrast, Th17 cells acquired cytotoxic potential only after antigenic stimulation and conversion to IFN-γ producing Th1 phenotype.
Conclusions: Our data demonstrate that both Th1 and Th17 lineages possess the ability to induce CNS autoimmunity but can function with complementary as well as differential pathogenic mechanisms. We propose that Th17-like cells producing IL-17 are required for the generation of atypical EAE whereas IFN-γ producing Th1 cells induce classical EAE.
Conflict of interest statement
Figures







References
-
- Ferber I, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, et al. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5–7. - PubMed
-
- Willenborg DO, Fordham SA, Bernard CCA, Cowden WB, Ramshaw IA. IFN-γ plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. - PubMed
-
- Zhang G-X, Gran B, Yu S, Li J, Siglienti I, et al. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170:2153–2160. - PubMed
-
- Gran B, Zhang G-X, Yu S, Chen X-H, Ventura ES, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: Evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

