Cancer-stromal interactions in scirrhous gastric carcinoma
- PMID: 21209779
- PMCID: PMC2990484
- DOI: 10.1007/s12307-010-0036-5
Cancer-stromal interactions in scirrhous gastric carcinoma
Abstract
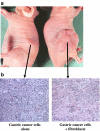
Fibroblasts play an important role in the progression, growth and spread of gastric cancers. Cancer-stroma interactions have been especially evident in the scirrhous type of gastric carcinoma. Fibroblasts are associated with the cancer progression at the primary and metastatic site. The proliferative and invasive ability of scirrhous gastric cancer cells are closely associated with the growth factors produced by organ-specific fibroblasts. Fibroblasts are therefore a key determinant in the malignant progression of gastric cancer and represent an important target for cancer therapies.
Keywords: Fibroblasts; Growth factor; Interaction; Microenvironment; Scirrhous gastric cancer; Stromal cells.
Figures




References
-
- Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ. 2004;157:327–349. - PubMed
LinkOut - more resources
Full Text Sources
Medical
