Stereotypical chronic lymphocytic leukemia B-cell receptors recognize survival promoting antigens on stromal cells
- PMID: 21209908
- PMCID: PMC3012720
- DOI: 10.1371/journal.pone.0015992
Stereotypical chronic lymphocytic leukemia B-cell receptors recognize survival promoting antigens on stromal cells
Abstract
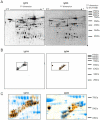
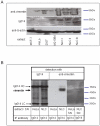
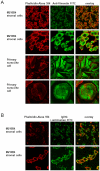
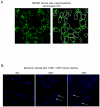
Chronic lymphocytic leukemia (CLL) is the most common leukemia in the Western world. Survival of CLL cells depends on their close contact with stromal cells in lymphatic tissues, bone marrow and blood. This microenvironmental regulation of CLL cell survival involves the stromal secretion of chemo- and cytokines as well as the expression of adhesion molecules. Since CLL survival may also be driven by antigenic stimulation through the B-cell antigen receptor (BCR), we explored the hypothesis that these processes may be linked to each other. We tested if stromal cells could serve as an antigen reservoir for CLL cells, thus promoting CLL cell survival by stimulation through the BCR. As a proof of principle, we found that two CLL BCRs with a common stereotyped heavy chain complementarity-determining region 3 (previously characterized as "subset 1") recognize antigens highly expressed in stromal cells--vimentin and calreticulin. Both antigens are well-documented targets of autoantibodies in autoimmune disorders. We demonstrated that vimentin is displayed on the surface of viable stromal cells and that it is present and bound by the stereotyped CLL BCR in CLL-stroma co-culture supernatant. Blocking the vimentin antigen by recombinant soluble CLL BCR under CLL-stromal cell co-culture conditions reduces stroma-mediated anti-apoptotic effects by 20-45%. We therefore conclude that CLL BCR stimulation by stroma-derived antigens can contribute to the protective effect that the stroma exerts on CLL cells. This finding sheds a new light on the understanding of the pathobiology of this so far mostly incurable disease.
Conflict of interest statement
Figures
Similar articles
-
Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling.Nature. 2012 Sep 13;489(7415):309-12. doi: 10.1038/nature11309. Nature. 2012. PMID: 22885698
-
The Traf2DNxBCL2-tg Mouse Model of Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Recapitulates the Biased IGHV Gene Usage, Stereotypy, and Antigen-Specific HCDR3 Selection of Its Human Counterpart.Front Immunol. 2021 Apr 12;12:627602. doi: 10.3389/fimmu.2021.627602. eCollection 2021. Front Immunol. 2021. PMID: 33912159 Free PMC article.
-
Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells.Blood. 2005 Jun 15;105(12):4820-7. doi: 10.1182/blood-2004-07-2669. Epub 2005 Feb 22. Blood. 2005. PMID: 15728130
-
B cell receptor and antigens in CLL.Adv Exp Med Biol. 2013;792:1-24. doi: 10.1007/978-1-4614-8051-8_1. Adv Exp Med Biol. 2013. PMID: 24014290 Review.
-
[Biological characteristics and clinical significance of stereotyped B-cell receptor in chronic lymphocytic leukemia].Zhonghua Xue Ye Xue Za Zhi. 2024 Feb 14;45(2):197-202. doi: 10.3760/cma.j.cn121090-20230718-00012. Zhonghua Xue Ye Xue Za Zhi. 2024. PMID: 38604800 Free PMC article. Review. Chinese.
Cited by
-
Novel Agents and Emerging Strategies for Targeting the B-Cell Receptor Pathway in CLL.Mediterr J Hematol Infect Dis. 2012;4(1):e2012067. doi: 10.4084/MJHID.2012.067. Epub 2012 Oct 9. Mediterr J Hematol Infect Dis. 2012. PMID: 23170196 Free PMC article.
-
Proliferative Signals in Chronic Lymphocytic Leukemia; What Are We Missing?Front Oncol. 2020 Oct 8;10:592205. doi: 10.3389/fonc.2020.592205. eCollection 2020. Front Oncol. 2020. PMID: 33134182 Free PMC article. Review.
-
The Number of Overlapping AID Hotspots in Germline IGHV Genes Is Inversely Correlated with Mutation Frequency in Chronic Lymphocytic Leukemia.PLoS One. 2017 Jan 26;12(1):e0167602. doi: 10.1371/journal.pone.0167602. eCollection 2017. PLoS One. 2017. PMID: 28125682 Free PMC article.
-
Immunological aspects in chronic lymphocytic leukemia (CLL) development.Ann Hematol. 2012 Jul;91(7):981-96. doi: 10.1007/s00277-012-1460-z. Epub 2012 Apr 12. Ann Hematol. 2012. PMID: 22526361 Free PMC article. Review.
-
Distinct transcriptional control in major immunogenetic subsets of chronic lymphocytic leukemia exhibiting subset-biased global DNA methylation profiles.Epigenetics. 2012 Dec 1;7(12):1435-42. doi: 10.4161/epi.22901. Epub 2012 Nov 15. Epigenetics. 2012. PMID: 23154584 Free PMC article.
References
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. - PubMed
-
- Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet. 2008;371:1017–1029. - PubMed
-
- Matutes E, Owusu-Ankomah K, Morilla R, Garcia Marco J, Houlihan A, et al. The immunological profile of B-cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia. 1994;8:1640–1645. - PubMed
-
- Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell'Aquila M, et al. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. - PubMed
-
- Burger JA, Kipps TJ. Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leuk Lymphoma. 2002;43:461–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

