Oral methylthioadenosine administration attenuates fibrosis and chronic liver disease progression in Mdr2-/- mice
- PMID: 21209952
- PMCID: PMC3012093
- DOI: 10.1371/journal.pone.0015690
Oral methylthioadenosine administration attenuates fibrosis and chronic liver disease progression in Mdr2-/- mice
Abstract
Background: Inflammation and fibrogenesis are directly related to chronic liver disease progression, including hepatocellular carcinoma (HCC) development. Currently there are few therapeutic options available to inhibit liver fibrosis. We have evaluated the hepatoprotective and anti-fibrotic potential of orally-administered 5'-methylthioadenosine (MTA) in Mdr2(-/-) mice, a clinically relevant model of sclerosing cholangitis and spontaneous biliary fibrosis, followed at later stages by HCC development.
Methodology: MTA was administered daily by gavage to wild type and Mdr2(-/-) mice for three weeks. MTA anti-inflammatory and anti-fibrotic effects and potential mechanisms of action were examined in the liver of Mdr2(-/-) mice with ongoing fibrogenesis and in cultured liver fibrogenic cells (myofibroblasts).
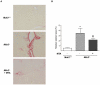
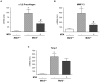
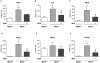
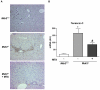
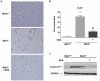
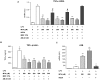
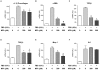
Principal findings: MTA treatment reduced hepatomegaly and liver injury. α-Smooth muscle actin immunoreactivity and collagen deposition were also significantly decreased. Inflammatory infiltrate, the expression of the cytokines IL6 and Mcp-1, pro-fibrogenic factors like TGFβ2 and tenascin-C, as well as pro-fibrogenic intracellular signalling pathways were reduced by MTA in vivo. MTA inhibited the activation and proliferation of isolated myofibroblasts and down-regulated cyclin D1 gene expression at the transcriptional level. The expression of JunD, a key transcription factor in liver fibrogenesis, was also reduced by MTA in activated myofibroblasts.
Conclusions/significance: Oral MTA administration was well tolerated and proved its efficacy in reducing liver inflammation and fibrosis. MTA may have multiple molecular and cellular targets. These include the inhibition of inflammatory and pro-fibrogenic cytokines, as well as the attenuation of myofibroblast activation and proliferation. Downregulation of JunD and cyclin D1 expression in myofibroblasts may be important regarding the mechanism of action of MTA. This compound could be a good candidate to be tested for the treatment of (biliary) liver fibrosis.
Conflict of interest statement
Figures








References
-
- Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–6. - PubMed
-
- Elsharkawy AM, Mann DA. Nuclear factor-κB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007;46:590–597. - PubMed
-
- Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol. 2005;45:605–628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

