NEK1 mutations cause short-rib polydactyly syndrome type majewski
- PMID: 21211617
- PMCID: PMC3014367
- DOI: 10.1016/j.ajhg.2010.12.004
NEK1 mutations cause short-rib polydactyly syndrome type majewski
Abstract
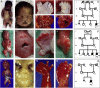
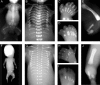
Defects of ciliogenesis have been implicated in a wide range of human phenotypes and play a crucial role in signal transduction and cell-cycle coordination. We used homozygosity mapping in two families with autosomal-recessive short-rib polydactyly syndrome Majewski type to identify mutations in NEK1 as an underlying cause of this lethal osteochondrodysplasia. NEK1 encodes a serine/threonine kinase with proposed function in DNA double-strand repair, neuronal development, and coordination of cell-cycle-associated ciliogenesis. We found that absence of functional full-length NEK1 severely reduces cilia number and alters ciliar morphology in vivo. We further substantiate a proposed digenic diallelic inheritance of ciliopathies by the identification of heterozygous mutations in NEK1 and DYNC2H1 in an additional family. Notably, these findings not only increase the broad spectrum of ciliar disorders, but suggest a correlation between the degree of defective microtubule or centriole elongation and organization and the severity of the resulting phenotype.
Figures



References
-
- Rohatgi R., Milenkovic L., Scott M.P. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. - PubMed
-
- Satir P., Christensen S.T. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 2007;69:377–400. - PubMed
-
- Rosenbaum J.L., Witman G.B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002;3:813–825. - PubMed
-
- Cole D.G., Snell W.J. SnapShot: Intraflagellar transport. Cell. 2009;137:784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

