Absence of Tec family kinases interleukin-2 inducible T cell kinase (Itk) and Bruton's tyrosine kinase (Btk) severely impairs Fc epsilonRI-dependent mast cell responses
- PMID: 21212279
- PMCID: PMC3059023
- DOI: 10.1074/jbc.M110.165613
Absence of Tec family kinases interleukin-2 inducible T cell kinase (Itk) and Bruton's tyrosine kinase (Btk) severely impairs Fc epsilonRI-dependent mast cell responses
Abstract
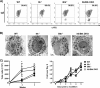
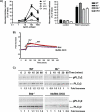
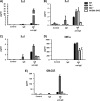
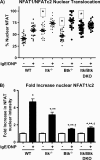
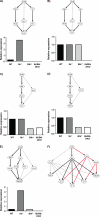
Mast cells are critical effector cells in the pathophysiology of allergic asthma and other IgE-mediated diseases. The Tec family of tyrosine kinases Itk and Btk serve as critical signal amplifiers downstream of antigen receptors. Although both kinases are expressed and activated in mast cells following FcεRI stimulation, their individual contributions are not clear. To determine whether these kinases play unique and/or complementary roles in FcεRI signaling and mast cell function, we generated Itk and Btk double knock-out mice. Analyses of these mice show decreased mast cell granularity and impaired passive systemic anaphylaxis responses. This impaired response is accompanied by a significant elevation in serum IgE in Itk/Btk double knock-out mice. In vitro analyses of bone marrow-derived mast cells (BMMCs) indicated that Itk/Btk double knock-out BMMCs are defective in degranulation and cytokine secretion responses downstream to FcεRI activation. These responses were accompanied by a significant reduction in PLCγ2 phosphorylation and severely impaired calcium responses in these cells. This defect also results in altered NFAT1 nuclear localization in double knock-out BMMCs. Network analysis suggests that although they may share substrates, Itk plays both positive and negative roles, while Btk primarily plays a positive role in mast cell FcεRI-induced cytokine secretion.
Figures



References
-
- Kawakami Y., Yao L., Tashiro M., Gibson S., Mills G. B., Kawakami T. (1995) J. Immunol. 155, 3556–3562 - PubMed
-
- Schmidt U., Abramova A., Boucheron N., Eckelhart E., Schebesta A., Bilic I., Kneidinger M., Unger B., Hammer M., Sibilia M., Valent P., Ellmeier W. (2009) Eur. J. Immunol. 39, 3228–3238 - PubMed
-
- Forssell J., Sideras P., Eriksson C., Malm-Erjefält M., Rydell-Törmänen K., Ericsson P., Erjefält J. (2005) Am. J. Respir. Cell Mol. Biol. 32, 511–520 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

