Global tissue revolutions in a morphogenetic movement controlling elongation
- PMID: 21212324
- PMCID: PMC3153412
- DOI: 10.1126/science.1199424
Global tissue revolutions in a morphogenetic movement controlling elongation
Abstract
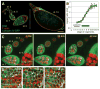
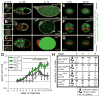
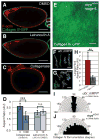
Polarized cell behaviors drive axis elongation in animal embryos, but the mechanisms underlying elongation of many tissues remain unknown. Eggs of Drosophila undergo elongation from a sphere to an ellipsoid during oogenesis. We used live imaging of follicles (developing eggs) to elucidate the cellular basis of egg elongation. We find that elongating follicles undergo repeated rounds of circumferential rotation around their long axes. Follicle epithelia mutant for integrin or collagen IV fail to rotate and elongate, which results in round eggs. We present evidence that polarized rotation is required to build a polarized, fibrillar extracellular matrix (ECM) that constrains tissue shape. Thus, global tissue rotation is a morphogenetic behavior that uses planar polarity information in the ECM to control tissue elongation.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

