Integrative systems models of cardiac excitation-contraction coupling
- PMID: 21212390
- PMCID: PMC3074965
- DOI: 10.1161/CIRCRESAHA.110.223578
Integrative systems models of cardiac excitation-contraction coupling
Abstract
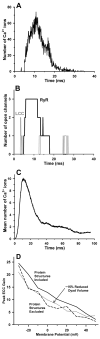
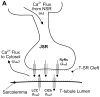
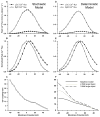
Excitation-contraction coupling in the cardiac myocyte is mediated by a number of highly integrated mechanisms of intracellular Ca²(+) transport. The complexity and integrative nature of heart cell electrophysiology and Ca²(+) cycling has led to an evolution of computational models that have played a crucial role in shaping our understanding of heart function. An important emerging theme in systems biology is that the detailed nature of local signaling events, such as those that occur in the cardiac dyad, have important consequences at higher biological scales. Multiscale modeling techniques have revealed many mechanistic links between microscale events, such as Ca²(+) binding to a channel protein, and macroscale phenomena, such as excitation-contraction coupling gain. Here, we review experimentally based multiscale computational models of excitation-contraction coupling and the insights that have been gained through their application.
Figures






References
-
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Boston: Kluwer; 2001.
-
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. - PubMed
-
- Balaban RS, Heineman FW. Control of mitochondrial respiration in the heart in vivo. Mol Cell Biochem. 1989;89:191–197. - PubMed
-
- Williams RS, Rosenberg P. Calcium-dependent gene regulation in myocyte hypertrophy and remodeling. Cold Spring Harb Symp Quant Biol. 2002;67:339–344. - PubMed
-
- Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

