Alternans and arrhythmias: from cell to heart
- PMID: 21212392
- PMCID: PMC3076605
- DOI: 10.1161/CIRCRESAHA.110.223586
Alternans and arrhythmias: from cell to heart
Abstract
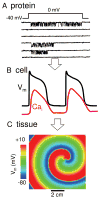
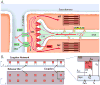
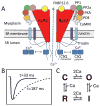
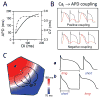
The goal of systems biology is to relate events at the molecular level to more integrated scales from organelle to cell, tissue, and living organism. Here, we review how normal and abnormal excitation-contraction coupling properties emerge from the protein scale, where behaviors are dominated by randomness, to the cell and tissue scales, where heart has to beat with reliable regularity for a lifetime. Beginning with the fundamental unit of excitation-contraction coupling, the couplon where L-type Ca channels in the sarcolemmal membrane adjoin ryanodine receptors in the sarcoplasmic reticulum membrane, we show how a network of couplons with 3 basic properties (random activation, refractoriness, and recruitment) produces the classic physiological properties of excitation-contraction coupling and, under pathophysiological conditions, leads to Ca alternans and Ca waves. Moving to the tissue scale, we discuss how cellular Ca alternans and Ca waves promote both reentrant and focal arrhythmias in the heart. Throughout, we emphasize the qualitatively novel properties that emerge at each new scale of integration.
Figures




References
-
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262(5134):740–744. - PubMed
-
- Lipp P, Niggli E. A hierarchical concept of cellular and subcellular Ca(2+)-signalling. Progress in Biophysics and Molecular Biology. 1996;65(3):265–296. - PubMed
-
- Eisner DA, Diaz ME, Li Y, O'Neill SC, Trafford AW. Stability and instability of regulation of intracellular calcium. Exp Physiol. 2005;90(1):3–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

