The role of HER-3 expression in the prediction of clinical outcome for advanced colorectal cancer patients receiving irinotecan and cetuximab
- PMID: 21212430
- PMCID: PMC3228051
- DOI: 10.1634/theoncologist.2010-0119
The role of HER-3 expression in the prediction of clinical outcome for advanced colorectal cancer patients receiving irinotecan and cetuximab
Abstract
Preclinical data suggested that, in the presence of human epidermal growth factor receptor (HER)-3-altered activation, colorectal cancer cells may escape anti-epidermal growth factor receptor (EGFR)-mediated cell death. HER-3 overexpression may then represent a key factor for resistance to anti-EGFR antibodies in colorectal cancer. The aim of our analysis was to investigate a possible correlation between HER-3 expression and clinical outcome in wild-type K-RAS advanced colorectal cancer patients receiving cetuximab and irinotecan. We retrospectively analyzed immunoreactivity for HER-3 in wild-type K-RAS advanced colorectal cancer patients receiving irinotecan and cetuximab. Eighty-four advanced wild-type K-RAS colorectal cancer patients were available for HER-3 analysis. Forty patients (48%) had a HER-3(-) colorectal tumor, whereas the remaining 44 cases (52%) were deemed HER-3(+). In patients with HER-3(-) and HER-3(+) tumors, we observed a partial response in 17 (42%) and eight (18%) patients respectively; progressive disease occurred in 11 (35%) and 26 (53%) patients with HER-3(-) and HER-3(+) tumors, respectively (p = .003). The median progression-free survival time was 6.3 months in patients with HER-3(-) tumors and 2.8 months for those who had HER-3-overexpressing tumors (p < .0001). The median overall survival time was 13.6 months in patients showing HER-3(-) tumors and 10.5 months for those who had HER-3-expressing tumors (p = .01). HER-3 proved to be a predictive factor for clinical outcome in wild-type K-RAS colorectal cancer patients treated with cetuximab. Combined HER-3 and K-RAS analysis may represent an effective strategy for better selection of responding colorectal cancer patients.
Conflict of interest statement
Section Editor
Section Editor
Section Editor
Reviewer “A” discloses no financial relationships.
Reviewer “B” discloses no financial relationships.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. On the basis of disclosed information, all conflicts of interest have been resolved.
Figures
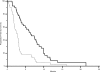

References
-
- Goldberg RM, Rothenberg ML, Van Cutsem E, et al. The continuum of care: A paradigm for the management of metastatic colorectal cancer. The Oncologist. 2007;12:38–50. - PubMed
-
- Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. J Clin Oncol. 2007;357:2040–2048. - PubMed
-
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. - PubMed
-
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. - PubMed
-
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

