Presence of reactive microglia and neuroinflammatory mediators in a case of frontotemporal dementia with P301S mutation
- PMID: 21212632
- PMCID: PMC3214942
- DOI: 10.1159/000322228
Presence of reactive microglia and neuroinflammatory mediators in a case of frontotemporal dementia with P301S mutation
Abstract
Background: Recent findings, showing the presence of an inflammatory process in the brain of transgenic mice expressing P301S mutated human tau protein, indicate that neuroinflammation may contribute to tau-related degeneration in frontotemporal dementia and parkinsonism linked to chromosome 17 with tau mutations (FTDP-17T).
Objective: To investigate the occurrence of neuroinflammatory changes in the brain of a patient affected by FTDP-17T associated with the P301S mutation and showing a frontotemporal dementia phenotype as well as in the brain of a patient affected by another FTDP-17T phenotype: multiple system tauopathy with presenile dementia.
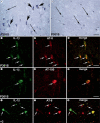
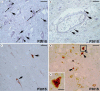
Methods: We used immunohistochemical methods to visualize activated microglia, interleukin-1b (IL-1b)-, cyclooxygenase-2 (COX-2)-expressing cells.
Results: In the brain of the patient with the P301S mutation, a strong neuroinflammatory reaction was present. Activated microglia/infiltrating macrophages expressing the cluster of differentiation 68 and major histocampatibility complex class II cell surface receptors, encoded by the human leukocyte antigen DP-DQ-DR, were detected in the cortex and hippocampus. IL-1b and COX-2 expression were induced in neuronal and glial cells. These neuroinflammatory changes were different from those observed in the brain of the patient bearing the +3 mutation, where macrophage infiltration was absent, microglial cells displayed an earlier stage of activation and COX-2 was not detected.
Conclusions: Our findings suggest that microglial activation and the production of proinflammatory mediators by phospho-tau-positive neurons and glial cells may differentially contribute to neuronal death and disease progression in neurodegenerative tauopathies.
Copyright © 2011 S. Karger AG, Basel.
Figures


References
-
- Wszolek ZK, Tsuboi Y, Farrer M, Uitti RJ, Hutton ML. Hereditary tauopathies and parkinsonism. Adv Neurol. 2003;91:153–163. - PubMed
-
- Baba Y, Baker MC, Le Ber I, Brice A, Maeck L, Kohlhase J, Yasuda M, Stoppe G, Bugiani O, Sperfeld AD, Tsuboi Y, Uitti RJ, Farrer MJ, Ghetti B, Hutton ML, Wszolek ZK. Clinical and genetic features of families with frontotemporal dementia and parkinsonism linked to chromosome 17 with a P301S tau mutation. J Neural Transm. 2007;114:947–950. - PubMed
-
- Gasparini L, Terni B, Spillantini MG. Frontotemporal dementia with tau pathology. Neurodegener Dis. 2007;4:236–253. - PubMed
-
- Bugiani O, Murrell JR, Giaccone G, Hasegawa M, Ghigo G, Tabaton M, Morbin M, Primavera A, Carella F, Solaro C, Grisoli M, Savoiardo M, Spillantini MG, Tagliavini F, Goedert M, Ghetti B. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol. 1999;58:667–677. - PubMed
-
- Bugiani O. FTDP-17: phenotypical heterogeneity within P301S. Ann Neurol. 2000;48:126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

