Spread deficits in initiation, speed and accuracy of horizontal and vertical automatic saccades in dementia with lewy bodies
- PMID: 21212841
- PMCID: PMC3015135
- DOI: 10.3389/fneur.2010.00138
Spread deficits in initiation, speed and accuracy of horizontal and vertical automatic saccades in dementia with lewy bodies
Abstract
Background: Mosimann et al. (2005) reported prolongation of saccade latency of prosaccades in dementia with Lewy body (DLB). The goal of this study is to go further examining all parameters, such as rates of express latency, but also accuracy and velocity of saccades, and their variability.
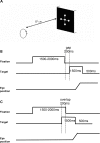
Methods: We examined horizontal and vertical saccades in 10 healthy elderly subjects and 10 patients with DLB. Two tasks were used: the gap (fixation target extinguishes prior to target onset) and the overlap (fixation stays on after target onset). Eye movements were recorded with the Eyelink II eye tracker.
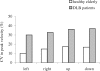
Results: The main findings were: (1) as for healthy, latencies were shorter in the gap than in the overlap task (a gap effect); (2) for both tasks latency of saccades was longer for DLB patients and for all directions; (3) express latency in the gap task was absent for large majority of DLB patients while such saccades occurred frequency for controls; (4) accuracy and peak velocity were lower in DLB patients; (5) variability of all parameters was abnormally high in DLB patients.
Conclusions: Abnormalities of all parameters, latency, accuracy and peak velocity reflect spread deficits in cortical-subcortical circuits involved in the triggering and execution of saccades.
Keywords: Lewy body; accuracy; latency; saccades; variability; velocity.
Figures




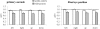
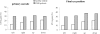


References
-
- Armstrong I. T., Chan F., Riopelle R. J., Munoz D. P. (2002). Control of saccades in Parkinson's disease. Brain Cogn. 49, 198–201 - PubMed
-
- Bell A. H., Everling S., Munoz D. P. (2000). Influence of stimulus eccentricity and direction on characteristics of pro- and antisaccades in non-human primates. J. Neurophysiol. 84, 2595–2604 - PubMed
-
- Boxer A. L., Geschwind M. D., Belfor N., Gorno-Tempini M. L., Schauer G. F., Miller B. L., Weiner M. W., Rosen H. J. (2006). Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch. Neurol. 63, 81–8610.1001/archneur.63.1.81 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

