Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels
- PMID: 21213095
- PMCID: PMC3108180
- DOI: 10.1007/s12630-010-9419-9
Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels
Abstract
Purpose: The Cys-loop ligand-gated ion channel superfamily is a major group of neurotransmitter-activated receptors in the central and peripheral nervous system. The superfamily includes inhibitory receptors stimulated by γ-aminobutyric acid (GABA) and glycine and excitatory receptors stimulated by acetylcholine and serotonin. The first part of this review presents current evidence on the location of the anesthetic binding sites on these channels and the mechanism by which binding to these sites alters their function. The second part of the review addresses the basis for this selectivity, and the third part describes the predictive power of a quantitative allosteric model showing the actions of etomidate on γ-aminobutyric acid type A receptors (GABA(A)Rs).
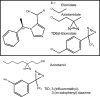
Principal findings: General anesthetics at clinical concentrations inhibit the excitatory receptors and enhance the inhibitory receptors. The location of general anesthetic binding sites on these receptors is being defined by photoactivable analogues of general anesthetics. The receptor studied most extensively is the muscle-type nicotinic acetylcholine receptor (nAChR), and progress is now being made with GABA(A)Rs. There are three categories of sites that are all in the transmembrane domain: 1) within a single subunit's four-helix bundle (intrasubunit site; halothane and etomidate on the δ subunit of AChRs); 2) between five subunits in the transmembrane conduction pore (channel lumen sites; etomidate and alcohols on nAChR); and 3) between two subunits (subunit interface sites; etomidate between the α1 and β2/3 subunits of the GABA(A)R).
Conclusions: These binding sites function allosterically. Certain conformations of a receptor bind the anesthetic with greater affinity than others. Time-resolved photolabelling of some sites occurs within milliseconds of channel opening on the nAChR but not before. In GABA(A)Rs, electrophysiological data fit an allosteric model in which etomidate binds to and stabilizes the open state, increasing both the fraction of open channels and their lifetime. As predicted by the model, the channel-stabilizing action of etomidate is so strong that higher concentrations open the channel in the absence of agonist. The formal functional paradigm presented for etomidate may apply to other potent general anesthetic drugs. Combining photolabelling with structure-function mutational studies in the context of allosteric mechanisms should lead us to a more detailed understanding of how and where these important drugs act.
Figures


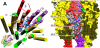
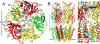


References
-
- Jurd R, Arras M, Lambert S, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB Journal. 2003;17:250–2. - PubMed
-
- Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–24. - PubMed
-
- Hemmings HC, Jr., Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. - PubMed
-
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. - PubMed
-
- Grasshoff C, Drexler B, Rudolph U, Antkowiak B. Anaesthetic drugs: linking molecular actions to clinical effects. Curr Pharm Des. 2006;12:3665–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

