[3+2] cycloadditions of aryl cyclopropyl ketones by visible light photocatalysis
- PMID: 21214249
- PMCID: PMC3033486
- DOI: 10.1021/ja107849y
[3+2] cycloadditions of aryl cyclopropyl ketones by visible light photocatalysis
Abstract
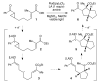
We report a new method for the formal [3+2] reaction of aryl cyclopropyl ketones with olefins to generate highly substituted cyclopentane ring systems. The key initiation step in this process is the one-electron reduction of the ketone to the corresponding radical anion, which is accomplished using a photocatalytic system comprising Ru(bpy)(3)(2+), La(OTf)(3), and TMEDA.
References
-
-
For a recent review, see: De Simone F, Waser J. Synthesis. 2009:3353–3374.
-
-
- Corey EJ, Chaykovsky M. J. Am. Chem. Soc. 1965;87:1353–1364.
- Doyle MP. Chem. Rev. 1986;86:919–939.
- Lebel H, Marcoux JF, Molinaro C, Charette AB. Chem. Rev. 2003;103:977–1050. - PubMed
-
-
For recent reviews of donor-acceptor cyclopropane chemistry, see: Reissig H-U, Zimmer R. Chem. Rev. 2003;103:1151–1196. Yu M, Pagenkopf BL. Tetrahedron. 2005;61:321–347. Campbell MJ, Johnson JS, Parsons AT, Pohlhaus PD, Sanders SD. J. Org. Chem. 2010;75:6317–3290. 2010.
-
-
-
For recent reviews of methylene cyclopropane chemistry, see: Binger P, Büch HM. Top. Curr. Chem. 1987;135:77–151. Brandi A, Goti A. Chem. Rev. 1998;98:589–635. Brandi A, Cicchi S, Cordero FM, Goti A. Chem. Rev. 2003;103:1213–1269.
-
-
- Alper PB, Meyers C, Lerchner A, Siegel DR, Carreira EM. Angew. Chem. Int. Ed. 1999;38:3186–3189. - PubMed
- Lautens M, Han W. J. Am. Chem. Soc. 2002;124:6312–6316. - PubMed
- Carson CA, Kerr MA. Chem. Soc. Rev. 2009;38:3051–3060. - PubMed
- Jiao L, Ye S, Yu Z-X. J. Am. Chem. Soc. 2008;130:7178–7179. - PubMed
- Jiao L, Lin M, Yu Z-X. Chem. Commun. 2010;46:1059–1061. - PubMed
- Li Q, Jiang G-J, Jiao L, Yu Z-X. Org. Lett. 2010;12:1332–1335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources