APP processing in Alzheimer's disease
- PMID: 21214928
- PMCID: PMC3022812
- DOI: 10.1186/1756-6606-4-3
APP processing in Alzheimer's disease
Abstract
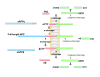
An important pathological feature of Alzheimer's disease (AD) is the presence of extracellular senile plaques in the brain. Senile plaques are composed of aggregations of small peptides called β-amyloid (Aβ). Multiple lines of evidence demonstrate that overproduction/aggregation of Aβ in the brain is a primary cause of AD and inhibition of Aβ generation has become a hot topic in AD research. Aβ is generated from β-amyloid precursor protein (APP) through sequential cleavages first by β-secretase and then by γ-secretase complex. Alternatively, APP can be cleaved by α-secretase within the Aβ domain to release soluble APPα and preclude Aβ generation. Cleavage of APP by caspases may also contribute to AD pathologies. Therefore, understanding the metabolism/processing of APP is crucial for AD therapeutics. Here we review current knowledge of APP processing regulation as well as the patho/physiological functions of APP and its metabolites.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical