Dynamics between stem cells, niche, and progeny in the hair follicle
- PMID: 21215372
- PMCID: PMC3050564
- DOI: 10.1016/j.cell.2010.11.049
Dynamics between stem cells, niche, and progeny in the hair follicle
Abstract
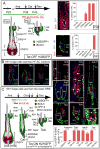
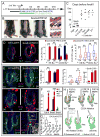
Here, we exploit the hair follicle to define the point at which stem cells (SCs) become irreversibly committed along a differentiation lineage. Employing histone and nucleotide double-pulse-chase and lineage tracing, we show that the early SC descendents en route to becoming transit-amplifying cells retain stemness and slow-cycling properties and home back to the bulge niche when hair growth stops. These become the primary SCs for the next hair cycle, whereas initial bulge SCs become reserves for injury. Proliferating descendents further en route irreversibly lose their stemness, although they retain many SC markers and survive, unlike their transit-amplifying progeny. Remarkably, these progeny also home back to the bulge. Combining purification and gene expression analysis with differential ablation and functional experiments, we define critical functions for these non-SC niche residents and unveil the intriguing concept that an irreversibly committed cell in an SC lineage can become an essential contributor to the niche microenvironment.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





Comment in
-
There and back again: hair follicle stem cell dynamics.Cell Stem Cell. 2011 Jan 7;8(1):8-9. doi: 10.1016/j.stem.2010.12.018. Cell Stem Cell. 2011. PMID: 21211777
References
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. - PubMed
-
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. - PubMed
-
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

