Presenilin-dependent receptor processing is required for axon guidance
- PMID: 21215373
- PMCID: PMC3034090
- DOI: 10.1016/j.cell.2010.11.053
Presenilin-dependent receptor processing is required for axon guidance
Abstract
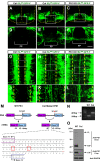
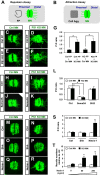
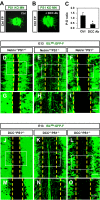
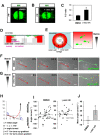
The Alzheimer's disease-linked gene presenilin is required for intramembrane proteolysis of amyloid-β precursor protein, contributing to the pathogenesis of neurodegeneration that is characterized by loss of neuronal connections, but the role of Presenilin in establishing neuronal connections is less clear. Through a forward genetic screen in mice for recessive genes affecting motor neurons, we identified the Columbus allele, which disrupts motor axon projections from the spinal cord. We mapped this mutation to the Presenilin-1 gene. Motor neurons and commissural interneurons in Columbus mutants lacking Presenilin-1 acquire an inappropriate attraction to Netrin produced by the floor plate because of an accumulation of DCC receptor fragments within the membrane that are insensitive to Slit/Robo silencing. Our findings reveal that Presenilin-dependent DCC receptor processing coordinates the interplay between Netrin/DCC and Slit/Robo signaling. Thus, Presenilin is a key neural circuit builder that gates the spatiotemporal pattern of guidance signaling, thereby ensuring neural projections occur with high fidelity.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. - PubMed
-
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. - PubMed
-
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. - PubMed
-
- De Strooper B, Annaert W. Novel Research Horizons for Presenilins and gamma-Secretases in Cell Biology and Disease. Annu Rev Cell Dev Biol - PubMed
-
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

